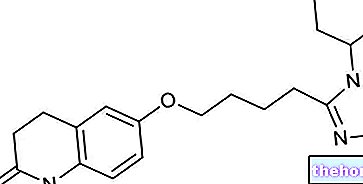
Ingrédients actifs : Oxolamine (citrate d'oxolamine), Propifénazone
UNIPLUS Adultes 250 mg + 350 mg Suppositoires
UNIPLUS Enfants 125 mg + 150 mg Suppositoires
UNIPLUS Petite Enfance 60 mg + 50 mg Suppositoires
Pourquoi Uniplus est-il utilisé ? Pourquoi est-ce?
CATÉGORIE PHARMACOTHERAPEUTIQUE : Analgésique-antipyrétique
INDICATIONS THÉRAPEUTIQUES : Traitement de l'inflammation des voies aériennes supérieures (sinusite, amygdalite, pharyngite, laryngite, trachéobronchite) ; otite; chaires; parodontite; traitement symptomatique des affections grippales.
Contre-indications Quand Uniplus ne doit pas être utilisé
: Hypersensibilité connue à l'un des composants ; enfants de moins de 2 mois; granulocytopénie; porphyrie aiguë intermittente; insuffisance de glucose-6-phosphate déshydrogénase.
Précautions d'emploi Ce qu'il faut savoir avant de prendre Uniplus
En raison de la présence de propiphénazone, l'administration de doses élevées ou de traitements prolongés avec le produit peut provoquer des lésions hématologiques chez les sujets hypersensibles. Bien qu'« aucun effet tératogène n'ait été démontré chez l'animal, chez la femme enceinte le produit ne doit être administré besoin réel, sous surveillance médicale stricte. Chez les femmes qui allaitent, il convient de garder à l'esprit la possibilité que les ingrédients actifs du produit soient excrétés avec le lait.
Interactions Quels médicaments ou aliments peuvent modifier l'effet d'Uniplus
: Aucune interaction négative n'a été rapportée avec d'autres médicaments couramment utilisés en thérapie liée aux indications thérapeutiques
Avertissements Il est important de savoir que :
: Lors de traitements prolongés, des contrôles périodiques de la formule sanguine sont recommandés.
Gardez le produit hors de la portée des enfants.
Posologie et mode d'utilisation Comment utiliser Uniplus : Posologie
- Suppositoires adultes : 1 suppositoire 2 à 3 fois par jour selon avis du médecin.
- Suppositoires enfants - enfants de plus de 2 ans : 1 suppositoire 2 à 3 fois par jour selon avis du médecin.
- Suppositoires petite enfance - enfants de 6 mois à 2 ans : 1 suppositoire 2 à 3 fois par jour selon l'âge et l'avis du médecin.
Effets secondaires Quels sont les effets secondaires d'Uniplus
Rarement, des éruptions cutanées allergiques peuvent survenir.
Signalez tout effet indésirable non décrit dans cette notice à votre médecin ou votre pharmacien.
Expiration et conservation
ATTENTION ne pas utiliser le médicament après la date de péremption indiquée sur l'emballage
A conserver à une température ne dépassant pas 30°C.
COMPOSITION:
- Suppositoires adultes - chaque suppositoire contient des ingrédients actifs : citrate d'oxolamine 0,25 g ; propiphénazone g 0,35
- Suppositoires pour enfants - chaque suppositoire contient des ingrédients actifs: citrate d'oxolamine 0,125 g; propiphénazone g 0,15
- Suppositoires pour la petite enfance - chaque microsuppositoire contient des ingrédients actifs : citrate d'oxolamine g 0,06 ; propiphénazone g 0,05 Excipients : glycérides semi-synthétiques.
FORMULAIRE PHARMACEUTIQUE
- Suppositoires adultes : boîte de 10 suppositoires
- Suppositoires enfants : boîte de 10 suppositoires
- Suppositoires petite enfance : boîte de 10 suppositoires.
Notice d'emballage source : AIFA (Agence italienne des médicaments). Contenu publié en janvier 2016. Les informations présentes peuvent ne pas être à jour.
Pour avoir accès à la version la plus récente, il est conseillé d'accéder au site Internet de l'AIFA (Agence Italienne du Médicament). Avis de non-responsabilité et informations utiles.
01.0 DÉNOMINATION DU MÉDICAMENT
UNIPLUS
02.0 COMPOSITION QUALITATIVE ET QUANTITATIVE
Chaque suppositoire contient :
Adultes: citrate d'oxolamine g 0,250; propiphénazone g 0,350;
Enfants: citrate d'oxolamine 0,125 g; propiphénazone g 0,150;
Petite enfance: citrate d'oxolamine g 0,060; propiphénazone g 0,050.
03.0 FORME PHARMACEUTIQUE
Suppositoires pour adultes ; suppositoires Enfants; suppositoires Petite enfance.
04.0 INFORMATIONS CLINIQUES
04.1 Indications thérapeutiques
Traitement de l'inflammation des voies respiratoires supérieures (sinusite, amygdalite, pharyngite, trachéobronchite). Otite. Formes rhumatismales. Chaires, parodontite. Traitement symptomatique des affections grippales.
04.2 Posologie et mode d'administration
Suppositoires :
Adultes: 1 suppositoire deux à trois fois par jour, selon l'avis du médecin.
Enfants (plus de deux ans): 1 suppositoire deux à trois fois par jour selon l'avis du médecin.
Petite enfance (Enfants âgés de 6 mois à 2 ans) : 1 suppositoire deux à trois fois par jour, selon l'âge et l'avis du médecin.
04.3 Contre-indications
Hypersensibilité déjà connue aux composants. Utilisation chez les enfants de moins de 2 mois. Granulocytopénie, porphyrie aiguë intermittente, insuffisance en glucose-6-phosphate déshydrogénase.
04.4 Mises en garde spéciales et précautions d'emploi appropriées
En raison de la présence de propiphénazone, des doses élevées et des traitements prolongés avec le produit peuvent endommager le sang chez les sujets hypersensibles.
Lors de traitements prolongés, des contrôles périodiques de la formule sanguine sont recommandés. Tenir hors de portée des enfants.
04.5 Interactions avec d'autres médicaments et autres formes d'interactions
L'utilisation simultanée d'autres médicaments n'a montré aucune interférence négative avec Uniplus.
04.6 Grossesse et allaitement
Bien qu'aucun effet tératogène n'ait été démontré chez l'animal, chez la femme enceinte, le médicament ne doit être utilisé qu'en cas de besoin réel et sous surveillance médicale stricte ; chez la femme qui allaite, il est nécessaire d'envisager la possibilité que les principes actifs contenus dans le produit soient excrétés. avec du lait.
04.7 Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
La prise du médicament n'affecte pas la capacité de conduire ou d'utiliser d'autres machines.
04.8 Effets indésirables
Dans de rares cas, des éruptions cutanées allergiques peuvent survenir.
04.9 Surdosage
L'intoxication aiguë se manifeste par une anorexie, des nausées et des vomissements et, dans les cas les plus graves, par une détérioration de l'état général. Les mesures comprennent l'utilisation de cortisone, la diurèse forcée, l'hémodialyse.
05.0 PROPRIÉTÉS PHARMACOLOGIQUES
05.1 Propriétés pharmacodynamiques
Mécanisme d'action : Uniplus modifie positivement les composants de l'inflammation des bronches et des muqueuses des voies respiratoires, fluidifiant les sécrétions catarrhales et prévenant les complications.Cette gamme d'action est due à la formulation originale d'Uniplus qui comprend un antipyrétique-analgésique (propiphénazone) et un anti-inflammatoire des voies respiratoires caractérisé par une activité antitussive (oxolamine).
05.2 "Propriétés pharmacocinétiques
Uniplus est facilement absorbé après administration orale et rectale. Les substances constitutives sont réparties dans les différents tissus ; Oxolamina, cependant, montre un tropisme spécifique pour le système respiratoire. Les composants sont en grande partie éliminés dans l'urine après avoir été largement transformés dans l'organisme.
05.3 Données de sécurité précliniques
Toxicologie : Les tests toxicologiques sur différentes espèces animales ont montré que les deux substances composant Uniplus sont bien tolérées et n'ont pas d'action tératogène ou mutagène Activité : aux doses thérapeutiques Uniplus est capable d'exercer une action antipyrétique, anti-inflammatoire et analgésique.
06.0 INFORMATIONS PHARMACEUTIQUES
06.1 Excipients
Chaque suppositoire contient :
Adultes: glycérides semi-synthétiques 2 100 g.
Enfants: glycérides semi-synthétiques 1400 g.
Petite enfance: glycérides semi-synthétiques g 0,690.
06.2 Incompatibilité
Aucune incompatibilité n'a été constatée.
06.3 Durée de validité
Les suppositoires sont stables pendant 4 ans.
06.4 Précautions particulières de conservation
A conserver à une température ne dépassant pas 30°C.
06.5 Nature du conditionnement primaire et contenu de l'emballage
Boîte de 10 suppositoires Adultes
Boîte de 10 suppositoires Enfants
Boîte de 10 suppositoires Petite enfance
06.6 Instructions d'utilisation et de manipulation
-----
07.0 TITULAIRE DE L'AUTORISATION DE MISE SUR LE MARCHE
A.C.R. Angelini Francesco S.p.A. - Viale Amelia, 70 - 00181 ROME
08.0 NUMÉRO D'AUTORISATION DE MISE SUR LE MARCHÉ
Code:
suppositoires Adultes 020075040
suppositoires Enfants 020075065
suppositoires Petite enfance 020075089
Date de première commercialisation : 1/12/1962
09.0 DATE DE PREMIÈRE AUTORISATION OU DE RENOUVELLEMENT DE L'AUTORISATION
-----
10.0 DATE DE RÉVISION DU TEXTE
-----