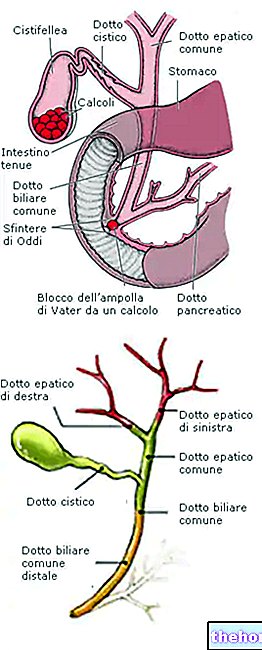
donnés par ceux qui ont récupéré du Covid-19. Ils peuvent être répliqués en quantités considérables grâce aux techniques de purification et d'expansion.
Les anticorps monoclonaux agissent avec le même mécanisme d'action que les anticorps polyclonaux.Les anticorps monoclonaux, en effet, possèdent une affinité hautement spécifique pour un certain type d'antigène et s'y fixent, permettant ainsi d'obtenir une réponse immunitaire marquée par rapport à cette toxine, protéine , médiateur chimique, cellule maligne ou agent pathogène qui est la cible du traitement. Ils sont essentiellement divisés en deux macro catégories :- Anticorps monoclonaux nus (c'est-à-dire non conjugués à d'autres molécules);
- Anticorps monoclonaux conjugués à des médicaments ou à des isotopes radioactifs.
Leur champ d'application, chez les patients Covid-19, a donné des résultats très satisfaisants, notamment chez les patients chez qui l'infection dure depuis quelques jours, et qui ne sont pas hospitalisés.Le médicament, en effet, doit être administré de façon précoce. phase " d'infection, après environ 72 heures, dans une phase dans laquelle la charge virale est au plus haut niveau, et doit être combattue et neutralisée. L'objectif est de prévenir une évolution virulente de la maladie, en anticipant les symptômes les plus sévères.Un traitement par ces antiviraux pourrait être indiqué chez les patients plus à risque d'évolution négative de l'infection, donc les patients en âge avancé, en évaluant les cas en fonction des conditions. du système immunitaire ou d'autres maladies présentes.
, qui se déroule en une seule perfusion veineuse, doit dans tous les cas être réalisée par du personnel médical spécialisé.Certains de ces médicaments ont montré qu'ils réduisaient le risque hospitalier jusqu'à 70% si le traitement est effectué dans la phase initiale de la maladie .
Le coût du médicament est très élevé : une perfusion d'anticorps monoclonaux coûte de 1 000 à 2 000 euros.Une dépense qui semblerait justifiée si l'on considère que ces médicaments peuvent être une réponse efficace contre la charge virale et rendre ceux qui les reçoivent immunisés et non plus longtemps L'expérience a également montré que les effets secondaires graves sont comparables à ceux du placebo, précisément en raison du profil de sécurité élevé.
Voir les autres articles tag Thrombose - Anticorps