Ingrédients actifs : Mébendazole
VERMOX 100 mg comprimés
VERMOX 20 mg/ml suspension buvable
Les notices d'emballage Vermox sont disponibles pour les tailles d'emballage : - VERMOX 100 mg comprimés, VERMOX 20 mg/ml suspension buvable
- VERMOX 500 mg comprimés
Pourquoi Vermox est-il utilisé ? Pourquoi est-ce?
CATÉGORIE PHARMACOTHERAPEUTIQUE
VERMOX (mébendazole) appartient à la catégorie des médicaments anthelminthiques.
INDICATIONS THÉRAPEUTIQUES
Infestations par les oxyures, les ascaris, les trichures, les ankylostomes, les strongyloïdes, les ténias.Il a notamment une puissante activité contre de nombreux vers parasites (=helminthes) de l'homme appartenant aux classes des nématodes et des ténias.VERMOX est particulièrement actif contre :
- Enterobius vermicularis (oxyure)
- Ascaris lumbricoides (vers rond)
- Trichuris trichiura (trichure)
- Ancylostoma duodenale (ankylostome)
- Necator americanus (ankylostome)
- Strongyloides stercoralis (strongyloïde)
- Taenia spp. (ver solitaire)
Contre-indications Quand Vermox ne doit pas être utilisé
Hypersensibilité à la substance active ou à l'un des excipients VERMOX ne doit pas être administré en cas de grossesse connue ou suspectée, ni pendant l'allaitement.
Précautions d'emploi Quelles sont les informations à connaître avant de prendre Vermox
Utilisation chez les enfants de moins de 1 an : En l'absence de documentation complète chez les enfants de moins de 1 an et en raison de rapports sporadiques de convulsions dans ce groupe de patients, VERMOX ne doit être administré que dans le cas où l'infestation parasitaire interfère de manière significative avec l'état nutritionnel et le développement physique.
Les résultats d'une étude cas-témoins portant sur un événement aigu de syndrome de Stevens-Johnson/nécrolyse épidermique toxique (SJS/NET) ont suggéré une relation possible entre SJS/NET et l'utilisation concomitante de mébendazole et de métronidazole. Aucune autre information n'est disponible. sur ce type d'interaction.Par conséquent, l'utilisation concomitante de mébendazole et de métronidazole doit être évitée.
Interactions Quels médicaments ou aliments peuvent modifier l'effet de Vermox
Informez votre médecin ou votre pharmacien si vous avez récemment pris tout autre médicament, même sans ordonnance.
Un traitement concomitant par la cimétidine peut inhiber le métabolisme hépatique du mébendazole, entraînant une augmentation des concentrations plasmatiques du médicament, en particulier lors d'un traitement prolongé. Dans ce cas, il est recommandé de déterminer la concentration plasmatique de mébendazole et d'ajuster la posologie.
L'utilisation concomitante de mébendazole et de métronidazole doit être évitée.
Avertissements Il est important de savoir que :
La grossesse et l'allaitement
Demandez conseil à votre médecin ou votre pharmacien avant de prendre tout médicament.Le médicament ne doit pas être administré si vous êtes enceinte ou présumée être enceinte, ou si vous allaitez.
Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Aucune étude sur l'aptitude à conduire des véhicules et à utiliser des machines n'a été réalisée.
Dose, méthode et moment d'administration Comment utiliser Vermox : Posologie
Dosage
1. OXYURIASES : dose unique de 100 mg (un comprimé ou une cuillère doseuse contenant 5 ml de suspension) par voie orale.
Le cycle évolutif d'Enterobius, agent de l'oxyurose, est très court. Par conséquent, les risques de réinfestation sont très élevés, surtout dans les grandes communautés sociales. Pour ces raisons, il est recommandé de répéter le traitement après 2 à 4 semaines.
2. ASCARIDIOSE, TRICHOCÉPHALOSE, ANKYLOSTOMIE ET INFESTATIONS MIXTES : une dose de 100 mg (un comprimé, ou une cuillère doseuse de 5 ml de suspension) par voie orale deux fois par jour (matin et soir) Répéter le traitement pendant 3 jours consécutifs indépendamment de l'âge et poids du patient.
3. TÉNIASE ET STRONGYLODIASE :
Adultes : bien que de bons résultats aient été obtenus à des doses plus faibles, une dose de 200-300 mg (2-3 comprimés, soit 2-3 cuillères-mesure de 5 ml de suspension) par voie orale est recommandée, à répartir en deux prises quotidiennes (matin et soir) pendant trois jours consécutifs.
Enfant : une dose de 100 mg (un comprimé ou une cuillère doseuse de 5 ml de suspension) par voie orale deux fois par jour (matin et soir) pendant trois jours consécutifs Pour les enfants de moins de 1 an voir rubrique "MISE EN GARDE PARTICULIERES".
Mode d'administration
VERMOX se présente sous forme de comprimés et de suspension à usage oral. Une cuillère doseuse de suspension contient la même quantité de principe actif qu'un comprimé.
Les comprimés peuvent être avalés avec un peu d'eau ou croqués avec un repas. Le traitement ne nécessite pas de régime alimentaire particulier, ni l'utilisation de laxatifs.
Agiter la suspension avant utilisation.
Un dosage adapté à chaque indication permet d'éliminer tous les vers chez plus de 90 % des patients, même en cas d'infestations sévères ou mixtes.
Surdosage Que faire si vous avez pris trop de Vermox
En cas d'ingestion/prise accidentelle d'une dose excessive de VERMOX, prévenez immédiatement votre médecin ou rendez-vous à l'hôpital le plus proche.
Si plus que les quantités recommandées de VERMOX sont prises ou pendant des périodes prolongées, des problèmes sanguins, rénaux ou hépatiques peuvent survenir, dont certains peuvent être graves. La perte de cheveux peut également se produire, qui dans certains cas peut être permanente. Dans tous les cas, les traitements à long terme doivent être soigneusement surveillés par le médecin.
En cas de surdosage accidentel, des crampes abdominales, des nausées, des vomissements et des diarrhées peuvent survenir. Dans ce cas, vous devez contacter votre médecin qui vous donnera du charbon activé pour absorber la quantité de VERMOX encore présente dans votre estomac.
Bien que la durée maximale de traitement recommandée soit limitée à 3 jours seulement, de rares cas d'altérations réversibles de la fonction hépatique, d'hépatite et de neutropénie ont été rapportés chez des patients traités pour une maladie hydatique (= ecchinococcose) avec des doses élevées et pendant de longues périodes.
Il n'y a pas d'antidote spécifique. Un lavage gastrique peut être effectué pendant la première heure suivant l'ingestion. Du charbon activé peut être administré si nécessaire. Si vous avez des questions sur l'utilisation de VERMOX, demandez plus d'informations à votre médecin ou votre pharmacien.
Effets secondaires Quels sont les effets secondaires de Vermox
Comme tous les médicaments, VERMOX est susceptible d'avoir des effets indésirables, bien que tout le monde n'y soit pas sujet.
Les effets secondaires suivants ont été signalés pouvant survenir pendant le traitement par VERMOX :
- Vertiges
- Gêne et douleur abdominale, flatulence, diarrhée
- Démangeaison de la peau
- Urticaire
- Perte de cheveux qui dans certains cas pourrait être permanente
- Troubles du sang et du foie
- Problèmes rénaux pouvant survenir lors d'une utilisation prolongée de VERMOX à des doses sensiblement supérieures à celles recommandées (beaucoup plus élevées que celles normalement prescrites).
Contactez immédiatement votre médecin si l'un des symptômes suivants apparaît :
- Une maladie cutanée grave qui se manifeste par des éruptions cutanées, des cloques sur la peau et des ulcères dans la bouche, une inflammation des yeux ou dans la région anogénitale et de la fièvre
- Une réaction qui survient peu après l'administration et se caractérise par une éruption cutanée, des démangeaisons, un essoufflement et/ou un gonflement du visage.
- Une réaction d'hypersensibilité sévère survenant peu après l'administration qui peut être caractérisée par de l'urticaire, des démangeaisons, des bouffées vasomotrices, des évanouissements et des difficultés respiratoires, parmi les symptômes possibles.
- Des convulsions (convulsions) ont été rapportées, y compris chez les nouveau-nés. VERMOX ne doit être administré aux enfants de moins d'un an que si le médecin l'a spécifiquement prescrit.
Le respect des instructions contenues dans la notice réduit le risque d'effets indésirables.
Si l'un des effets indésirables devient grave ou si vous remarquez des effets indésirables non décrits dans cette notice, veuillez en informer votre médecin.
Expiration et conservation
Date de péremption : voir la date de péremption indiquée sur l'emballage.
Attention : ne pas utiliser le médicament après la date de péremption indiquée sur l'emballage.
La date fait référence au produit dans un emballage intact, correctement stocké.
Gardez ce médicament hors de la portée et de la vue des enfants.
Les médicaments ne doivent pas être jetés au tout à l'égout ou avec les ordures ménagères.Demandez à votre pharmacien comment éliminer les médicaments que vous n'utilisez plus.Cela contribuera à protéger l'environnement.
espace de rangement
Comprimés : le médicament ne nécessite pas de conditions particulières de conservation.
Suspension : A conserver à une température ne dépassant pas 25°C.
Fermeture à l'épreuve des enfants : (comprimés)
Blister opaque
Fermeture sécurité enfant : (suspension)
Bouteille en verre avec tasse à mesurer
A l'aide du gobelet doseur : VERSER LA SUSPENSION DANS LE "Creux INDIQUÉ PAR LA FLÈCHE SUR LA MESURE" (comme décrit sur le dessin)
Les trous du gobelet doseur permettent à la suspension de sortir si elle est versée par erreur du côté opposé à celui indiqué par la flèche
Composition et forme pharmaceutique
COMPOSITION
Un comprimé contient :
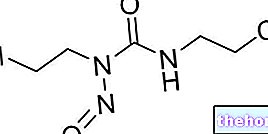
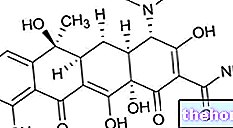
SUBSTANCE ACTIVE : mébendazole 100 mg.
EXCIPIENTS : amidon de maïs, saccharinate de sodium, laurylsulfate de sodium, jaune orangé, arôme orange, cellulose microcristalline, dioxyde de silice colloïdale, stéarate de magnésium, glycolate d'amidon, sel de sodium, talc, huile de coton hydrogénée.
Un ml de suspension buvable contient :
SUBSTANCE ACTIVE : mébendazole 20 mg.
EXCIPIENTS : cellulose microcristalline et carmellose de sodium, laurylsulfate de sodium, parahydroxybenzoate de méthyle, parahydroxybenzoate de propyle, acide citrique monohydraté, méthylcellulose, arôme banane, saccharose, eau purifiée.
FORME PHARMACEUTIQUE ET CONTENU
Comprimés de 100 mg - 6 comprimés
20 mg/ml suspension buvable - flacon de 30 ml
Notice d'emballage source : AIFA (Agence italienne des médicaments). Contenu publié en janvier 2016. Les informations présentes peuvent ne pas être à jour.
Pour avoir accès à la version la plus récente, il est conseillé d'accéder au site Internet de l'AIFA (Agence Italienne du Médicament). Avis de non-responsabilité et informations utiles.
01.0 DÉNOMINATION DU MÉDICAMENT
VERMOX 100 mg comprimés et 20 mg/ml suspension buvable
02.0 COMPOSITION QUALITATIVE ET QUANTITATIVE
Un comprimé contient :
Ingrédient actif : mébendazole 100 mg
Un ml de suspension pour usage oral contient :
Ingrédient actif : mébendazole 20 mg
Pour la liste complète des excipients, voir rubrique 6.1.
03.0 FORME PHARMACEUTIQUE
Comprimés de 100 mg
20 mg/ml suspension buvable
04.0 INFORMATIONS CLINIQUES
04.1 Indications thérapeutiques
Infestations par les oxyures, les ascaris, les trichures, les ankylostomes, les strongyloïdes, les ténias.
VERMOX (mébendazole) est un dérivé de synthèse du benzimidazole doté d'une puissante activité vermifuge contre les nématodes et les cestoïdes.
VERMOX est particulièrement actif chez l'homme contre :
• Enterobius vermicularis (oxyure)
• Ascaris lumbricoides (ascaride)
• Trichuris trichiura (trichure)
• Ancylostoma duodenale (ankylostome)
• Necator Americanus (ankylostome)
• Strongyloides stercolaris (strongioloide)
• Taenia spp. (ver solitaire)
Un dosage adapté à chaque indication permet d'éliminer tous les vers chez plus de 90 % des patients, même en cas d'infestations sévères ou mixtes.
04.2 Posologie et mode d'administration
Ossiurie : dose unique de 100 mg (un comprimé ou une cuillère-mesure de 5 ml de suspension).
Le cycle évolutif d'Enterobius, l'agent de l'oxyurose, est très court. Par conséquent, les risques de réinfection sont très élevés, en particulier dans les grandes communautés sociales. Pour ces raisons, il est recommandé de répéter le traitement après 2 à 4 semaines.
Ascaridiase, trichocéphalose, ankylostomes et infestations mixtes : une dose de 100 mg (un comprimé ou une cuillère doseuse de 5 ml de suspension) deux fois par jour (matin et soir), pendant trois jours consécutifs, quels que soient l'âge et le poids du patient.
Téniase et strongyloïdose
Adultes : bien que de bons résultats aient été obtenus à des doses plus faibles, une dose de 200-300 mg (deux-trois comprimés, ou deux-trois cuillères-mesure de 5 ml de suspension) est recommandée deux fois par jour (matin et soir), pendant trois jours.
Enfant : une dose de 100 mg (un comprimé ou une cuillère doseuse de 5 ml de suspension) deux fois par jour (matin et soir), pendant trois jours consécutifs.
Pour les enfants de moins d'un an, voir rubrique 4.4.
Les comprimés peuvent être avalés avec un peu d'eau ou croqués avec un repas. Le traitement ne nécessite pas de régime alimentaire particulier, ni l'utilisation de laxatifs.Agiter la suspension avant utilisation.
04.3 Contre-indications
VERMOX est contre-indiqué chez les personnes présentant une hypersensibilité à la substance active ou à l'un des excipients.
04.4 Mises en garde spéciales et précautions d'emploi appropriées
Au cours de l'expérience post-commercialisation avec VERMOX, des épisodes de convulsions ont été très rarement rapportés chez les enfants, y compris les enfants de moins d'un an (voir rubrique 4.8).VERMOX 100 mg ne doit être administré aux jeunes enfants que dans les cas où une infestation parasitaire interfère de manière significative avec l'état nutritionnel et le développement physique.
Les résultats d'une étude cas-témoins portant sur un événement aigu de syndrome de Stevens-Johnson/nécrolyse épidermique toxique (SJS/NET) ont suggéré une relation possible entre SJS/NET et l'utilisation concomitante de mébendazole et de métronidazole. Aucune autre information n'est disponible. sur ce type d'interaction.Par conséquent, l'utilisation concomitante de mébendazole et de métronidazole doit être évitée.
04.5 Interactions avec d'autres médicaments et autres formes d'interactions
Un traitement concomitant par la cimétidine peut inhiber le métabolisme hépatique du mébendazole, entraînant une augmentation des concentrations plasmatiques du médicament, en particulier lors de traitements prolongés. Dans ce cas, il est recommandé de déterminer la concentration plasmatique de mébendazole et d'ajuster la posologie.
L'utilisation concomitante de mébendazole et de métronidazole doit être évitée (voir rubrique 4.4).
04.6 Grossesse et allaitement
VERMOX ne doit pas être administré en cas de grossesse confirmée ou présumée, ni pendant l'allaitement.
04.7 Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Aucune étude sur l'aptitude à conduire des véhicules et à utiliser des machines n'a été réalisée.
04.8 Effets indésirables
Données d'études cliniques
L'innocuité de VERMOX a été évaluée chez 6276 sujets participant à 39 essais cliniques pour le traitement d'infestations parasitaires simples ou mixtes du tractus gastro-intestinal. Dans ces 39 études cliniques, aucun effet indésirable médicamenteux (EIM) n'a été signalé chez ≥1 % des sujets traités par VERMOX. Les effets indésirables des médicaments (EIM) rapportés par ≤1 % des sujets traités par VERMOX sont présentés dans le Tableau 1.
Données post-commercialisation
Les événements indésirables identifiés au cours de l'expérience post-commercialisation avec VERMOX (mébendazole) sont inclus dans le tableau 2. Dans chaque tableau, les fréquences sont rapportées selon la convention suivante :
Très fréquent (≥1 / 10)
Commun (≥1 / 100,
Peu fréquent (≥1 / 1 000 à
Rares (≥1 / 10 000,
Très rare (
Dans le tableau 2, les événements indésirables sont présentés par fréquence sur la base de notifications spontanées.
04.9 Surdosage
Chez les patients traités à des doses sensiblement plus élevées que celles recommandées ou pendant des périodes prolongées, les effets indésirables suivants ont été rarement rapportés : troubles réversibles de la fonction hépatique, hépatite, neutropénie et glomérulonéphrite. A l'exception de la glomérulonéphrite, ces effets indésirables ont également été rapportés chez des patients traités aux doses standard (voir rubrique 4.8.2).
Symptômes
En cas de surdosage accidentel, des crampes abdominales, des nausées, des vomissements et des diarrhées peuvent survenir.
Traitement
Il n'y a pas d'antidote spécifique. Un lavage gastrique peut être effectué pendant la première heure après l'ingestion. Du charbon activé peut être administré si nécessaire.
05.0 PROPRIÉTÉS PHARMACOLOGIQUES
05.1 Propriétés pharmacodynamiques
Classe pharmacothérapeutique : Anthelminthiques pour administration orale, dérivés du benzimidazole.
Code ATC : P02CA01
Pour les indications thérapeutiques (voir rubrique 4.1), le mébendazole agit localement dans la lumière de l'estomac en interférant avec les formations de tubuline cellulaire dans l'intestin des parasites.Le mébendazole se lie spécifiquement à la tubuline et provoque des modifications dégénératives ultrastructurales de l'intestin. Ce processus se traduit par un blocage de l'absorption du glucose par les parasites avec un dérèglement de leurs fonctions digestives qui conduit ainsi à un processus autolytique.
Il n'y a aucune preuve que VERMOX est efficace dans le traitement de la cysticercose.
05.2 Propriétés pharmacocinétiques
Après administration orale de 0,1 mg/kg de poids corporel de mébendazole radiomarqué, une absorption minimale par le tractus gastro-intestinal a été observée. Aux doses thérapeutiques normales, la biodisponibilité est faible, car le médicament subit un effet de premier passage métabolique marqué et également en raison de sa faible solubilité. Environ 90 % de la fraction absorbée est liée aux protéines plasmatiques.
05.3 Données de sécurité précliniques
Pour une administration aiguë :
DL50 (rat albinos, per os) : 1500 mg/kg ; poumon
DL50 (souris albinos, per os) : 1500 mg/kg
Pour une administration prolongée :
Rat albinos per os (28 jours) : dose max n'ayant pas provoqué d'altérations 200 mg/kg/jour
Rat albinos per os (180 jours) : dose max n'ayant pas provoqué d'altérations 40 mg/kg/jour
Chien per os (180 jours) : dose max n'ayant pas provoqué d'altérations 40 mg/kg/jour
Absence de manifestations histologiques suspectées de cancérogenèse.
Toxicité fœtale :
Rat albinos, per os : augmentation des résorptions (30 mg/kg/jour)
Lapin, par voie orale : dose max n'ayant pas provoqué d'altérations : 30 mg/kg/jour.
06.0 INFORMATIONS PHARMACEUTIQUES
06.1 Excipients
Un comprimé contient :
Excipients : amidon de maïs, saccharinate de sodium, laurylsulfate de sodium, jaune orangé, arôme orangé, cellulose microcristalline, silice colloïdale, stéarate de magnésium, glycolate d'amidon, sel de sodium, talc, huile de coton hydrogénée.
Un ml de suspension pour usage oral contient :
Excipients : cellulose microcristalline et carmellose de sodium, laurylsulfate de sodium, para-hydroxybenzoate de méthyle, para-hydroxybenzoate de propyle, acide citrique monohydraté, méthylcellulose, arôme banane, saccharose, eau purifiée.
06.2 Incompatibilité
VERMOX 100 mg comprimés : sans objet.
VERMOX 20 mg/ml suspension buvable : en l'absence d'études d'incompatibilité, ce médicament ne doit pas être mélangé avec d'autres produits.
06.3 Durée de validité
Comprimés à 100 mg : 3 ans
20 mg/ml suspension buvable : 4 ans
06.4 Précautions particulières de conservation
Comprimés : le médicament ne nécessite pas de conditions particulières de conservation
Suspension : A conserver à une température ne dépassant pas 25°C
06.5 Nature du conditionnement primaire et contenu de l'emballage
6 comprimés de 100 mg sous blister opaque.
Flacon de 30 ml de suspension buvable.
06.6 Instructions d'utilisation et de manipulation
La solution doit être agitée avant utilisation.
A l'aide du gobelet doseur : VERSER LA SUSPENSION DANS LE "Creux INDIQUÉ PAR LA FLÈCHE SUR LA MESURE"
Les trous du gobelet doseur permettent à la suspension de sortir si elle est versée par erreur du côté opposé à celui indiqué par la flèche.
07.0 TITULAIRE DE L'AUTORISATION DE MISE SUR LE MARCHE
JANSSEN-CILAG SpA
via M. Buonarroti 23
20093 Cologno Monzese (Milan)
08.0 NUMÉRO D'AUTORISATION DE MISE SUR LE MARCHÉ
AIC n. 023821010 - Comprimés à 100 mg 6 comprimés
AIC n. 023821022 - 20 mg/ml suspension buvable flacon de 30 ml
09.0 DATE DE PREMIÈRE AUTORISATION OU DE RENOUVELLEMENT DE L'AUTORISATION
Juin 2000 / Juin 2005
10.0 DATE DE RÉVISION DU TEXTE
Décision AIFA du 19 avril 2010