Ingrédients actifs : Chlorchinaldol, Diflucortolone
IMPETEX 1% + 0,1% CRÈME
Indications Pourquoi utiliser Impetex ? Pourquoi est-ce?
Groupe pharmacothérapeutique
Corticoïdes à usage cutané, associés à des antiseptiques. Indications Toutes affections cutanées, tant inflammatoires qu'allergiques, accompagnées d'infections bactériennes, fongiques ou mixtes, pour lesquelles une corticothérapie locale est indiquée. Affections cutanées avec complications bactériennes et/ou fongiques : eczéma nummulaire, eczéma et dermatite de contact, eczéma vulgaire (phase aiguë et chronique), eczéma séborrhéique, eczéma variqueux (pas directement sur l'ulcère), eczéma des enfants, eczéma anal, dyshidrose et dyshidrose eczéma, psoriasis, microbides, eczématisme.
Infections bactériennes à composante inflammatoire intense : pyodermite, folliculite, impétigo.
Dermatomycose causée par des dermatophytes, des levures et des champignons de type lévulo, accompagnée d'une inflammation aiguë ou avec le tableau clinique dominé par une eczématisation secondaire (teigne, candidose, pityriasis versicolor).
Impetex est également indiqué pour prévenir les infections bactériennes et/ou fongiques dans les affections cutanées de nature inflammatoire ou allergique.
Contre-indications Quand Impetex ne doit pas être utilisé
Hypersensibilité aux substances actives ou à l'un des excipients.
Infections tuberculeuses et virales de la peau à traiter (herpès, varicelle, etc.), acné rosacée, ulcères cutanés.
La préparation est contre-indiquée pour un usage ophtalmique.
Précautions d'emploi Quelles sont les informations à connaître avant de prendre Impetex
L'utilisation, surtout prolongée, de produits à usage topique peut donner lieu à des phénomènes de sensibilisation, dans ce cas il est nécessaire d'interrompre le traitement et d'instaurer une thérapie adéquate.
Interactions Quels médicaments ou aliments peuvent modifier l'effet d'Impetex
Informez votre médecin ou votre pharmacien si vous avez récemment pris d'autres médicaments, même ceux sans ordonnance.
Il n'y a pas d'interactions médicamenteuses et d'incompatibilités possibles connues.
Avertissements Il est important de savoir que :
L'application percutanée de corticoïdes dans le traitement des dermatoses étendues et/ou de longue durée peut déterminer des phénomènes secondaires d'absorption systémique (syndrome de Cushing, inhibition de l'axe hypothalamo-hypophysaire). Cette occurrence est plus fréquente chez l'enfant et en cas de pansement occlusif. Chez le nourrisson et l'enfant de moins de 4 ans, il est recommandé de ne pas poursuivre le traitement au-delà de 3 semaines, en particulier si les zones à traiter sont recouvertes de couches ou de culottes en plastique, car la peau se plisse et la couche peut faire office de pansement. occlusif. Dans le traitement des maladies chroniques nécessitant des thérapies prolongées, si un effet thérapeutique favorable a été obtenu, il sera conseillé de réduire la posologie et la fréquence des applications au minimum nécessaire pour contrôler les symptômes et éviter les rechutes, en suspendant l'utilisation de la préparation comme dès que possible.
Pendant le traitement, il est nécessaire de surveiller l'état du patient afin de détecter les premiers signes et symptômes d'un excès de stéroïdes (asthénie, hypertension, troubles électrolytiques, etc.). Dans tous les cas, il est conseillé de limiter l'utilisation de stéroïdes topiques à de courtes durées.L'utilisation, surtout si prolongée, de produits topiques peut donner lieu à des phénomènes de sensibilisation. Dans ce cas, il est nécessaire d'interrompre le traitement et d'instaurer une thérapie adéquate.
L'utilisation prolongée du produit peut favoriser le développement de micro-organismes non sensibles à l'agent chimiothérapeutique présent dans la préparation elle-même. Dans ce cas, des mesures thérapeutiques appropriées doivent être prises. En cas d'application sur le visage, éviter que la préparation n'entre en contact avec contact avec les yeux. "L'utilisation, surtout si prolongée, de produits à usage topique peut donner lieu à des phénomènes de sensibilisation. Dans ce cas, le traitement doit être interrompu et une thérapie appropriée instituée.
La grossesse et l'allaitement
Demandez conseil à votre médecin ou votre pharmacien avant de prendre tout médicament.
L'application locale de corticoïdes à des animaux de laboratoire gravides peut induire l'apparition de malformations fœtales.
La transférabilité de cette découverte à l'espèce humaine n'est pas prouvée. Cependant, au cours des 3 premiers mois de la grossesse, les préparations topiques de corticoïdes ne doivent pas être utilisées en grande quantité ou pendant une longue période et généralement chez les femmes enceintes et dans la très petite enfance, la préparation ne doit être utilisée qu'en cas de besoin réel et sous surveillance directe. chèque du médecin.
Avertissements sur les excipients :
Ce médicament contient de l'alcool stéarylique, qui peut provoquer des réactions cutanées locales (par exemple, une dermatite de contact).
Dose, mode et heure d'administration Comment utiliser Impetex : Posologie
De par son excipient particulier (émulsion "huile dans eau", faible en gras) Impetex est particulièrement indiqué dans le traitement des lésions sécrétoires et dans les zones cutanées humides, telles que la région anale et la cavité axillaire, où il est conseillé d'utiliser une base avec une forte teneur en eau. . Impetex permet à la sécrétion de s'écouler et induit une évaporation rapide et un dessèchement de la peau. La préparation ne laisse aucune trace de graisse sur la peau et convient donc également à une application sur le visage et les zones cutanées découvertes.
Sauf prescription contraire, commencez le traitement en étalant la préparation en couche mince 2 à 3 fois par jour. Dès que le tableau clinique s'est amélioré, une seule application quotidienne suffit.
Les nourrissons et les enfants de moins de 4 ans ne doivent pas être traités pendant plus de trois semaines, en particulier dans les zones couvertes de couches.
Surdosage Que faire si vous avez pris trop d'Impetex
Il n'y a pas de cas connus de surdosage
Effets secondaires Quels sont les effets secondaires d'Impetex
Rougeur locale, œdème, desquamation, démangeaisons comme signes d'hypersensibilité au produit.
D'autres effets comprennent l'hypertrichose, l'éruption acnéiforme, l'atrophie cutanée, l'hypopigmentation, les télangiectasies, les stries, la fragilité vasculaire, le purpura et, après des traitements prolongés (surtout sur le visage), la dermatite pustuleuse rebond qui, étant sensible aux stéroïdes, ne se manifeste qu'au moment de la arrêt du traitement. L'utilisation prolongée et/ou à forte dose peut induire un syndrome d'excès avec hypertension artérielle, asthénie, adynamie, troubles du rythme cardiaque, hypokaliémie et alcalose métabolique.
Dans les traitements occlusifs, il faut garder à l'esprit que les films utilisés pour le pansement peuvent eux-mêmes être à l'origine de phénomènes de sensibilisation.
Des réactions d'hypersensibilité peuvent survenir chez les sujets prédisposés.
Il est conseillé de consulter votre médecin ou votre pharmacien en cas d'effets indésirables non mentionnés dans cette notice.
Expiration et conservation
Expiration : Voir la date d'expiration imprimée sur l'emballage
La date de péremption fait référence au produit dans un emballage intact, correctement stocké
. Avertissement : N'utilisez pas le médicament après la date de péremption indiquée sur l'emballage.
Les médicaments ne doivent pas être jetés au tout-à-l'égout ou avec les ordures ménagères.Demandez à votre pharmacien comment jeter les médicaments que vous n'utilisez plus.Cela contribuera à protéger l'environnement.
GARDER LE MÉDICAMENT HORS DE LA PORTÉE ET DE LA VUE DES ENFANTS
Composition
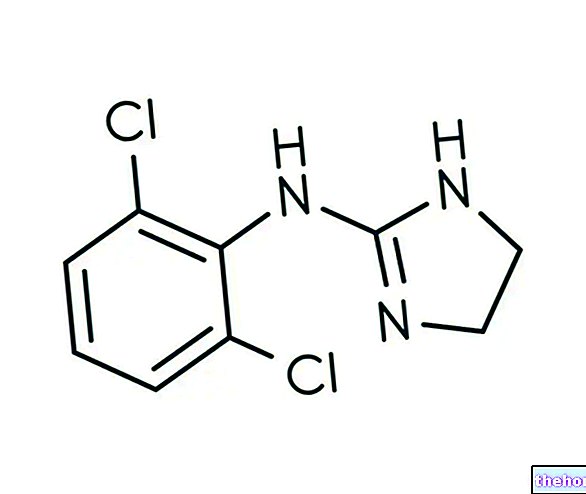
100 g de crème contiennent : 1 g de chlorchinaldol ; valérate de diflucortolone 0,1 g.
Excipients : monostéarate de polyéthylène glycol, alcool stéarylique, paraffine liquide, vaseline blanche, édétate de sodium, carbomère, hydroxyde de sodium, eau purifiée.
Forme et contenu pharmaceutiques
Crème, tube de 30 g.
Notice d'emballage source : AIFA (Agence italienne des médicaments). Contenu publié en janvier 2016. Les informations présentes peuvent ne pas être à jour.
Pour avoir accès à la version la plus à jour, il est conseillé d'accéder au site Internet de l'AIFA (Agence Italienne du Médicament). Avis de non-responsabilité et informations utiles.
01.0 DÉNOMINATION DU MÉDICAMENT
IMPETEX 1% + 0,1% CRÈME
02.0 COMPOSITION QUALITATIVE ET QUANTITATIVE
100 g de crème contiennent :
Principes actifs : chlorchinaldol 1 g, valérate de diflucortolone 0,1 g.
Excipients : Alcool stéarylique.
Pour la liste complète des excipients, voir rubrique 6.1.
03.0 FORME PHARMACEUTIQUE
Crème.
04.0 INFORMATIONS CLINIQUES
04.1 Indications thérapeutiques
Toutes affections cutanées, tant inflammatoires qu'allergiques, accompagnées d'infections bactériennes, fongiques ou mixtes, pour lesquelles une corticothérapie locale est indiquée.
Affections cutanées avec complications bactériennes et/ou fongiques : eczéma nummulaire, eczéma et dermatite de contact, eczéma vulgaire (phase aiguë et chronique), eczéma séborrhéique, eczéma variqueux (pas directement sur l'ulcère), eczéma des enfants, eczéma anal, dyshidrose et dyshidrose eczéma, psoriasis, microbides, eczématis Infections bactériennes à composante inflammatoire intense : pyodermite, folliculite, impétigo Dermatomycose causée par des dermatophytes, levures et champignons de type lévulo-like, accompagnée d'une inflammation aiguë ou avec le tableau clinique dominé par une eczématisation secondaire (teigne, candidose , pityriasis versicolor) Impetex est également indiqué pour prévenir les infections bactériennes et/ou fongiques dans les affections cutanées de nature inflammatoire ou allergique.
04.2 Posologie et mode d'administration
De par son excipient particulier (émulsion "huile dans eau", faible en gras) Impetex est particulièrement indiqué dans le traitement des lésions sécrétoires et dans les zones cutanées humides, telles que la région anale et la cavité axillaire, où il est conseillé d'utiliser une base avec une forte teneur en eau. . Impetex permet à la sécrétion de s'écouler et induit une évaporation rapide et un dessèchement de la peau. La préparation ne laisse aucune trace de graisse sur la peau et convient donc également à une application sur le visage et les zones cutanées découvertes.
Sauf prescription contraire, commencez le traitement en étalant la préparation en couche mince 2 à 3 fois par jour. Dès que le tableau clinique s'est amélioré, une seule application quotidienne suffit.
Les nourrissons et les enfants de moins de 4 ans doivent être traités pendant des périodes ne dépassant pas 3 semaines, en particulier lorsqu'ils sont appliqués dans des régions couvertes de couches.
04.3 Contre-indications
Hypersensibilité aux substances actives ou à l'un des excipients.
Infections tuberculeuses et virales de la peau à traiter (herpès, varicelle, etc.).
Acné rosacée.
Ulcères cutanés.
La préparation est contre-indiquée pour un usage ophtalmique.
04.4 Mises en garde spéciales et précautions d'emploi appropriées
L'application cutanée de corticoïdes dans le traitement des dermatoses étendues et/ou de longue durée, peut déterminer des phénomènes secondaires d'absorption systémique (syndrome de Cushing, inhibition de l'axe hypothalamo-hypophysaire). Cette occurrence est plus fréquente chez l'enfant et en cas de pansement occlusif. Chez le nourrisson et l'enfant de moins de 4 ans, il est recommandé de ne pas poursuivre le traitement au-delà de 3 semaines, en particulier si les zones à traiter sont recouvertes de couches ou de culottes en plastique car la peau se replie et la couche peut agir comme un pansement occlusif. .
Dans le traitement des maladies chroniques nécessitant des thérapies prolongées, si un effet thérapeutique favorable a été obtenu, il sera conseillé de réduire la posologie et la fréquence des applications au minimum nécessaire pour contrôler les symptômes et éviter les rechutes, en suspendant l'utilisation de la préparation comme dès que possible.
Pendant le traitement, il est nécessaire de surveiller l'état du patient afin de détecter les premiers signes et symptômes d'un excès de stéroïdes (asthénie, hypertension, troubles électrolytiques, etc.).
Dans tous les cas, il est conseillé de limiter l'utilisation de stéroïdes topiques à de courtes périodes de temps.
L'utilisation prolongée du produit peut favoriser le développement de micro-organismes non sensibles à l'agent chimiothérapeutique présent dans la préparation elle-même.Dans ce cas, des mesures thérapeutiques appropriées doivent être adoptées.
En cas d'application sur le visage, éviter que la préparation n'entre en contact avec les yeux. L'utilisation, surtout prolongée, de produits à usage topique peut donner lieu à des phénomènes de sensibilisation, dans ce cas il est nécessaire d'interrompre le traitement et d'instaurer une thérapie adéquate.
Les médicaments ne doivent pas être conservés à la portée des enfants.
Avertissements sur les excipients :
Ce médicament contient de l'alcool stéarylique, qui peut provoquer des réactions cutanées locales (par exemple, une dermatite de contact).
04.5 Interactions avec d'autres médicaments et autres formes d'interactions
Il n'y a pas d'interactions médicamenteuses possibles connues.
04.6 Grossesse et allaitement
L'application locale de corticostéroïdes à des animaux de laboratoire gravides peut induire l'apparition de malformations fœtales.La transférabilité de ce résultat à l'homme n'a pas été démontrée.
Cependant, au cours des trois premiers mois de la grossesse, les préparations topiques de corticostéroïdes ne doivent pas être utilisées en quantités élevées ou pendant une longue période et généralement chez les femmes enceintes et dans la très petite enfance, la préparation ne doit être utilisée qu'en cas de besoin réel et sous surveillance directe. chèque du médecin.
04.7 Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Impetex n'affecte pas l'aptitude à conduire des véhicules ou à utiliser des machines.
04.8 Effets indésirables
Localement rougeur, œdème, desquamation, démangeaisons comme signes d'hypersensibilité au produit. D'autres effets incluent une hypertrichose, des éruptions acnéiformes, une atrophie cutanée, une hypopigmentation, des télangiectasies, des stries, une fragilité vasculaire, un purpura et après des traitements prolongés (surtout sur le visage) une dermatite pustuleuse rebond qui, étant sensible aux stéroïdes, ne se manifeste qu'à l'arrêt du traitement. L'utilisation prolongée et/ou à fortes doses peut induire un syndrome d'excès avec hypertension artérielle, asthénie, adynamie, troubles du rythme cardiaque, hypokaliémie et alcalose métabolique.
Dans les traitements occlusifs, il faut garder à l'esprit que les films utilisés pour le pansement peuvent eux-mêmes provoquer des phénomènes de sensibilisation.
L'utilisation, surtout si prolongée des produits à usage topique, peut donner lieu à des phénomènes de sensibilisation, dans ce cas il est nécessaire d'interrompre le traitement et d'instaurer une thérapie adéquate.
Des réactions d'hypersensibilité peuvent survenir chez les sujets prédisposés.
04.9 Surdosage
Il n'y a pas de cas connus de surdosage.
05.0 PROPRIÉTÉS PHARMACOLOGIQUES
05.1 Propriétés pharmacodynamiques
Classe pharmacothérapeutique : corticoïde, association à un antiseptique.
Code ATC : D07BC04.
La crème Impetex contient un corticostéroïde topique, le valérinate de diflucortolone (DFV) à une concentration de 0,1% et un antimicrobien, le chlorchinaldol (CCD) à une concentration de 1%.
Le DFV est un corticostéroïde à application topique, à action anti-inflammatoire, antiprurigineuse et vasoconstrictrice. Les corticoïdes réduisent l'inflammation avec différents mécanismes, notamment en favorisant la synthèse d'un facteur (lipocortine) qui maintient sous contrôle l'enzyme (phospholipase A2), qui active la cascade de l'acide arachidonique, conduisant à la formation de facteurs phlogogènes, tels que les prostaglandines et les lipoperoxydes.
Le DFV présente une activité anti-inflammatoire 3 à 30 fois supérieure à celle des autres corticostéroïdes topiques de comparaison et une activité antiproliférative 10 fois supérieure à celle de la flucortolone.
Le CCD possède une activité antibactérienne et antifongique qui s'exerce in vitro sur les bactéries Gram positives, sur les principales bactéries Gram négatives, ainsi que sur les dermatophytes et les levures. Cet effet inhibiteur sur la croissance des microorganismes a été confirmé sur la peau humaine par le test du pansement occlusif selon Marples et Kligman. L'application, même répétée, du CCD ne favorise pas la sélection de souches bactériennes résistantes.
05.2 "Propriétés pharmacocinétiques
Appliqués sur la peau, les corticostéroïdes sont en grande partie retenus par la couche cornée et seule une petite partie atteint le derme où ils peuvent être absorbés. De nombreux facteurs peuvent favoriser une absorption plus évidente : la zone et l'extension de la peau à traiter, le type de lésion, la durée du traitement, tout pansement occlusif. A cet égard, il faut garder à l'esprit que certaines zones de la peau (visage, paupières, cheveux, scrotum) les absorbent plus facilement que d'autres (peau des genoux, des coudes, de la paume de la main et de la plante des pieds). Le DFV pénètre rapidement dans l'épiderme humain, atteignant sa concentration maximale dans les 4 heures suivant l'application.
Cette concentration est répandue dans les couches cutanées les plus superficielles. L'absorption systémique, après 7 heures d'application, est inférieure à 1 % de la dose initiale. La faible quantité absorbée dans la circulation est rapidement métabolisée (demi-vie plasmatique d'environ 4 heures) en au moins trois substances dégradantes qui sont rapidement et complètement éliminées par le rein sous forme conjuguée.
7 métabolites du DFV ont été identifiés dans les urines.
Le métabolisme intracutané du DFV, après application sur la peau humaine, consiste en une lente hydrolyse de la substance en diflucortolone et acide valérique (5-15 % de la dose appliquée pendant 7 heures).
Des investigations in vitro et in vivo, après application cutanée de diverses préparations contenant du CCD marqué au 14C chez des volontaires sains, ont montré que seule une quantité modeste de principe actif est absorbée : 5 à 15 % en 24 heures et dans différentes conditions. Après l'application cutanée de 25 g d'une préparation contenant 1% de CCD, la quantité absorbée varie de 10 à 35 mg en 24 heures.
Des recherches menées avec le DFV et le CCD ont montré que la présence du CCD ne modifie pas l'absorption du stéroïde et que ce dernier n'interfère pas négativement avec l'action antibactérienne du CCD.
05.3 Données de sécurité précliniques
La toxicité aiguë du DFV est négligeable (DL50 per os chez la souris > 4 g/kg). Des tests effectués avec des applications topiques à des concentrations égales à 0,5% ont confirmé l'absence d'une toxicité aiguë déterminable.Seulement après des applications prolongées chez le chien pendant 14 semaines de préparations à 0,1% à la dose de 100 mg/kg/jour oui des effets systémiques sont manifesté.
La toxicité aiguë du CCD a été évaluée chez la souris (DL50 per os 0,6-0,8 g/kg), le rat (DL50 2,9 g/kg) et le chien (DL50 1 g/kg).
L'application cutanée de doses de 2,5 g/kg d'une préparation à 10 % pendant dix jours chez le rat et treize jours chez le lapin n'a pas provoqué l'apparition d'effets toxiques notables.
La toxicité aiguë du DFV + CCD a été évaluée chez le rat (DL50 par voie orale > 35,9 g/kg). L'application continue pendant treize semaines chez le chien a provoqué l'apparition d'effets systémiques uniquement pour des doses supérieures à 500 mg/kg/jour.
Des effets embryotoxiques sont apparus chez le lapin et le rat uniquement pour des doses supérieures à 50 mg/kg/jour appliquées sur la peau, pendant la phase d'organogenèse, pendant plus de dix jours.
06.0 INFORMATIONS PHARMACEUTIQUES
06.1 Excipients
Monostéarate de polyéthylène glycol, alcool stéarylique, paraffine liquide, vaseline blanche, édétate de sodium, carbomère, hydroxyde de sodium, eau purifiée.
06.2 Incompatibilité
Aucune incompatibilité particulière n'est connue à ce jour.
06.3 Durée de validité
4 années.
06.4 Précautions particulières de conservation
Ce médicament ne nécessite pas de conditions particulières de conservation.
06.5 Nature du conditionnement primaire et contenu de l'emballage
Tube flexible en aluminium protégé intérieurement par de la laque et fermé par un bouchon à vis en plastique, contenu dans une boîte en carton, avec la notice.
06.6 Instructions d'utilisation et de manipulation
Pas d'instructions particulières.
07.0 TITULAIRE DE L'AUTORISATION DE MISE SUR LE MARCHE
Teofarma S.r.l. - Via F.lli Cervi, 8 - 27010 Valle Salimbene (PV)
08.0 NUMÉRO D'AUTORISATION DE MISE SUR LE MARCHÉ
Impetex 1% + 0,1% AIC crème n°024383022
09.0 DATE DE PREMIÈRE AUTORISATION OU DE RENOUVELLEMENT DE L'AUTORISATION
22/04/1981 / juin 2010
10.0 DATE DE RÉVISION DU TEXTE
Résolution AIFA du 28 janvier 2013