Ingrédients actifs : Oxerutina
VENORUTON 500 mg comprimés effervescents
VENORUTON 1000 mg comprimés effervescents
Les notices d'emballage de Venoruton sont disponibles pour les tailles d'emballage : - VENORUTON 500 mg comprimés effervescents, VENORUTON 1000 mg comprimés effervescents
- VENORUTON 1000 mg granulés pour solution buvable, VENORUTON 500 mg comprimés pelliculés, VENORUTON 2% gel
Pourquoi Venoruton est-il utilisé ? Pourquoi est-ce?
Qu'est-ce que c'est
VENORUTON est un vasoprotecteur à base d'oxérutine, une substance obtenue à partir des fleurs et des feuilles de Sophora Japonica.
Pourquoi est-il utilisé
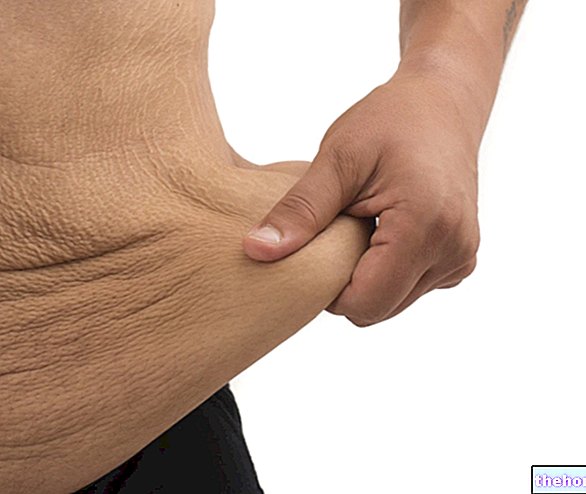
VENORUTON est indiqué dans le traitement des symptômes attribuables à l'insuffisance veineuse ; états de fragilité capillaire.
Contre-indications Quand Venoruton ne doit pas être utilisé
Hypersensibilité à l'oxérutine ou à l'un des excipients.
Les patients souffrant d'œdème des membres inférieurs dû à une maladie cardiaque, rénale ou hépatique ne doivent pas prendre Venoruton car l'effet de Venoruton n'est pas prouvé dans ces indications.
Venoruton n'est pas recommandé pour une utilisation chez les enfants.
Précautions d'emploi Quelles sont les informations à connaître avant de prendre Venoruton
Venoruton n'est pas recommandé pour une utilisation chez les enfants.
Interactions Quels médicaments ou aliments peuvent modifier l'effet de Venoruton
Informez votre médecin ou votre pharmacien si vous avez récemment pris tout autre médicament, même sans ordonnance.
A ce jour, aucune interaction spécifique de l'oxérutine avec d'autres médicaments n'a été rapportée.Les données de laboratoire sur une éventuelle modulation de l'activité des enzymes hépatiques par les composants de l'oxérutine (quercétine et rutine présentes à l'état de traces) sont discordantes.
Avertissements Il est important de savoir que :
Que faire pendant la grossesse et l'allaitement
Demandez conseil à votre médecin ou à votre pharmacien avant de prendre tout médicament. L'innocuité du médicament pendant la grossesse n'a pas été déterminée, il n'est donc pas recommandé pendant la grossesse. Dans les études animales, des traces d'oxérutine ont été trouvées dans le lait maternel. On suppose que les petites quantités d'orexutine qui passent dans le lait maternel peuvent être considérées comme sans pertinence clinique pour l'homme.
Les études chez l'animal n'ont montré aucun effet sur la fertilité après l'administration d'oxérutine.
Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Venoruton n'a aucun effet ou qu'un effet négligeable sur l'aptitude à conduire des véhicules et à utiliser des machines.
Dans de rares cas, de la fatigue et des étourdissements ont été rapportés chez des patients qui prenaient le produit. Il est déconseillé aux patients concernés de conduire ou d'utiliser des machines.
Informations importantes sur certains ingrédients
Ce médicament contient 10,15 mmol (396 mg) de potassium par comprimé. A prendre en considération chez les personnes dont la fonction rénale est réduite ou qui suivent un régime pauvre en potassium.
Ce médicament contient 3,56 mmol (82 mg) de sodium par comprimé. A prendre en considération chez les personnes suivant un régime pauvre en sodium
Posologie et mode d'utilisation Comment utiliser Venoruton : Posologie
Combien
Venoruton 1000 mg comprimés effervescents : 1 comprimé par jour.
Venoruton 500 mg comprimés effervescents : 2 comprimés par jour.
Attention : ne pas dépasser les doses indiquées sans avis médical.
Quand et pour combien de temps
Attention : n'utiliser que pour de courtes périodes de traitement. En cas d'exacerbation des symptômes, il est recommandé d'utiliser le produit par cycles.
Consultez votre médecin si le trouble survient à plusieurs reprises ou si vous remarquez un changement récent de ses caractéristiques.
Comme, comment
Chaque comprimé doit être soigneusement dissous dans un verre d'eau et pris avant ou pendant les repas.
Surdosage Que faire si vous avez pris trop de Venoruton
Aucun signe ou symptôme de surdosage de Venoruton n'a jamais été rapporté.
En cas d'ingestion/prise accidentelle d'un surdosage de Venoruton, prévenez immédiatement votre médecin ou rendez-vous à l'hôpital le plus proche.
Si vous avez des questions sur l'utilisation de Venoruton, demandez à votre médecin ou votre pharmacien
Effets secondaires Quels sont les effets secondaires de Venoruton
Comme tous les médicaments, VENORUTON est susceptible d'avoir des effets indésirables, bien que tout le monde n'y soit pas sujet.
Venoruton peut, dans de rares cas, provoquer des effets secondaires gastro-intestinaux ou des réactions cutanées telles que troubles gastro-intestinaux, flatulences, diarrhée, douleurs abdominales, maux d'estomac, dyspepsie, éruption cutanée, démangeaisons ou urticaire. La survenue d'étourdissements, de maux de tête, de bouffées de chaleur, de fatigue ou de réactions d'hypersensibilité telles que des réactions anaphylactoïdes est très rare.
Les effets indésirables sont énumérés ci-dessous par classification des systèmes d'organes et par fréquence. Les fréquences sont définies comme : très fréquente (≥ 1/10), fréquente (≥ 1/100 a
Troubles du système immunitaire
Très rare Réactions anaphylactoïdes
Très rare Réactions d'hypersensibilité
Troubles du système nerveux
Très rare Vertiges
Très rare Maux de tête
Pathologies vasculaires
Très rare Flushing
Problèmes gastro-intestinaux
Rare Troubles gastro-intestinaux
Flatulences rares
Rare Diarrhée
Rare Douleur abdominale
Rares maux d'estomac
Rare Dyspepsie
Affections de la peau et du tissu sous-cutané
Éruption cutanée rare
Prurit rare
Rare Urticaire
Troubles généraux et anomalies au site d'administration
Très rare Fatigue
Le respect des instructions contenues dans la notice réduit le risque d'effets indésirables.
Déclaration des effets secondaires
Si vous ressentez un quelconque effet indésirable, parlez-en à votre médecin ou votre pharmacien, y compris tout effet indésirable éventuel non mentionné dans cette notice. Vous pouvez également signaler les effets secondaires directement via le système national de déclaration à l'adresse "https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse". En signalant les effets secondaires, vous pouvez contribuer à fournir plus d'informations sur la sécurité de ce médicament.
Expiration et conservation
Expiration : voir la date d'expiration imprimée sur l'emballage.
La date de péremption fait référence au produit dans un emballage intact, correctement stocké. Attention : ne pas utiliser le médicament après la date de péremption indiquée sur l'emballage.
A conserver à une température inférieure à 30°C.
Conserver le récipient bien fermé pour le protéger de la lumière et de l'humidité.
Les médicaments ne doivent pas être jetés au tout à l'égout ou avec les ordures ménagères.Demandez à votre pharmacien comment éliminer les médicaments que vous n'utilisez plus.Cela contribuera à protéger l'environnement.
GARDER CE MEDICAMENT HORS DE LA VUE ET DE LA PORTEE DES ENFANTS.
Il est important de toujours disposer des informations sur le médicament, donc conservez à la fois la boîte et la notice.
Composition
Comprimés effervescents Venoruton 500 mg : Un comprimé contient : principe actif oxérutine 500 mg Excipients : acide citrique anhydre ; carbonate de potassium; bicarbonate de potassium; bicarbonate de sodium; macrogols; acésulfame de potassium; povidone; arôme orange (supporté sur maltodextrine), stéarate de magnésium.
Comprimés effervescents Venoruton 1000 mg : Un comprimé contient : principe actif oxérutine 1000 mg Excipients : acide citrique anhydre ; carbonate de potassium; bicarbonate de potassium; bicarbonate de sodium; macrogols; acésulfame de potassium; povidone; arôme orange (supporté sur maltodextrine), stéarate de magnésium.
A quoi ça ressemble
VENORUTON se présente sous la forme de comprimés effervescents de 500 mg ou 1000 mg, conditionnés dans un tube avec bouchon séchant. Le contenu du paquet est :
- 20 comprimés effervescents de 500 mg.
- 30 comprimés effervescents de 1000 mg (2 tubes de 15 comprimés chacun).
Notice d'emballage source : AIFA (Agence italienne des médicaments). Contenu publié en janvier 2016. Les informations présentes peuvent ne pas être à jour.
Pour avoir accès à la version la plus récente, il est conseillé d'accéder au site Internet de l'AIFA (Agence Italienne du Médicament). Avis de non-responsabilité et informations utiles.
01.0 DÉNOMINATION DU MÉDICAMENT
COMPRIMÉS EFFERVESCENTS VENORUTON
02.0 COMPOSITION QUALITATIVE ET QUANTITATIVE
Venoruton 500 mg comprimés effervescents: Un comprimé contient : principe actif oxérutine 500 mg. Excipients à effet notoire : carbonate de potassium, bicarbonate de potassium, bicarbonate de sodium, acésulfame de potassium.
Venoruton 1000 mg comprimés effervescents: Un comprimé contient : ingrédient actif oxérutine 1000 mg. Excipients à effet notoire : carbonate de potassium, bicarbonate de potassium, bicarbonate de sodium, acésulfame de potassium.
Pour la liste complète des excipients, voir rubrique 6.1
03.0 FORME PHARMACEUTIQUE
Comprimés effervescents
04.0 INFORMATIONS CLINIQUES
04.1 Indications thérapeutiques
VENORUTON est indiqué dans le traitement des symptômes attribuables à l'insuffisance veineuse ; états de fragilité capillaire.
04.2 Posologie et mode d'administration
Venoruton 1000 mg comprimés effervescents: 1 comprimé par jour.
Venoruton 500 mg comprimés effervescents: 2 comprimés par jour.
Chaque comprimé doit être soigneusement dissous dans un verre d'eau et pris avant ou pendant les repas.
04.3 Contre-indications
Hypersensibilité à la substance active ou à l'un des excipients mentionnés à la rubrique 6.1.
04.4 Mises en garde spéciales et précautions d'emploi appropriées
Informations importantes sur certains ingrédients
Ce médicament contient 10,15 mmol de potassium par comprimé. A prendre en considération chez les personnes dont la fonction rénale est réduite ou qui suivent un régime pauvre en potassium.
Ce médicament contient 3,56 mmol de sodium par comprimé. A prendre en considération chez les personnes suivant un régime pauvre en sodium.
04.5 Interactions avec d'autres médicaments et autres formes d'interactions
Aucun connu à ce jour.
04.6 Grossesse et allaitement
L'innocuité du médicament pendant la grossesse n'a pas été déterminée, il est donc conseillé de ne pas administrer le produit pendant la grossesse.
Il n'y a aucune restriction sur l'utilisation de la préparation pendant l'allaitement.
04.7 Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Aucun effet.
04.8 Effets indésirables
Même lorsque le traitement a été poursuivi pendant plusieurs mois, aucun effet indésirable notable n'a été signalé.
04.9 Surdosage
Aucun cas de surdosage n'a jamais été rapporté.
05.0 PROPRIÉTÉS PHARMACOLOGIQUES
05.1 Propriétés pharmacodynamiques
Groupe pharmacothérapeutique: substances protectrices capillaires - bioflavonoïdes;
Code ATC C05CA49.
L'oxérutine [O- (β-hydroxyéthyl) -rutosidea], principe actif contenu dans Venoruton, appartient à la famille des flavonoïdes et a une action antioxydante de par ses caractéristiques moléculaires.Il a également été démontré qu'elle a un tropisme pour l'endothélium veineux.
Sa présence au niveau capillaire permet, surtout lorsqu'en présence d'insuffisance veineuse la diminution de la vitesse du sang induit une hypoxie locale, d'antagoniser et d'intercepter les radicaux libres présents. Ces derniers sont connus pour être capables de provoquer des lésions cellulaires, point de départ de l'adhésion des granulocytes neutrophiles à l'endothélium avec déclenchement de la réaction inflammatoire qui se traduit par l'augmentation de la perméabilité capillaire et la formation d'œdèmes des membres inférieurs.
L'action antioxydante de l'oxérutine sur la membrane des cellules endothéliales et sur les érythrocytes de la microcirculation ainsi que l'effet inhibiteur sur la lipoxygénase des neutrophiles sont associés à la diminution de la perméabilité capillaire, à la réduction de la formation d'œdèmes, à un stimulus réduit à l'adhésion à l'endothélium pour les granulocytes neutrophiles et les plaquettes et à la restauration des caractéristiques rhéologiques des globules rouges au niveau capillaire.
En particulier, ces derniers phénomènes sont corrélés aux améliorations démontrées de la situation de l'oxygénation locale et du tonus veineux.
Les propriétés pharmacodynamiques spécifiques de l'oxérutine sont donc transférables également à des syndromes dont la pathogenèse est similaire à celle de l'insuffisance veineuse comme celle du plexus hémorroïdaire.
05.2 Propriétés pharmacocinétiques
Les comprimés effervescents Venoruton sont une forme pharmaceutique caractérisée par une dissolution rapide et une absorption relativement rapide de l'ingrédient actif. En effet, il présente l'avantage de permettre l'obtention d'une pharmacoémie élevée en peu de temps grâce à la rapidité avec laquelle il libère le principe actif dans les liquides biologiques. Absorbé dans le tractus gastro-intestinal, le médicament est principalement excrété par la voie biliaire.
05.3 Données de sécurité précliniques
La toxicologie de l'oxérutine [O- (β-hydroxyéthyl) -rutosidea] a été évaluée chez plusieurs espèces animales.
La DL50 chez le rat est comprise entre 24 000 et 27 000 mg/kg, selon la voie d'administration.
Dans les tests de toxicité chronique chez le rat, menés avec des doses de 2 850 mg/kg/jour pendant 90 jours, aucune action toxique du médicament n'a été trouvée.
Les tests de tératogenèse, de fertilité et de toxicité péri-postnatale n'ont révélé aucune anomalie chez les descendants.
06.0 INFORMATIONS PHARMACEUTIQUES
06.1 Excipients
Acide citrique anhydre; carbonate de potassium; bicarbonate de potassium; bicarbonate de sodium; macrogols; acésulfame de potassium; povidone; arôme orange (supporté sur maltodextrine), stéarate de magnésium.
06.2 Incompatibilité
Rien.
06.3 Durée de validité
4 années
06.4 Précautions particulières de conservation
A conserver à une température inférieure à 30°C. Conserver le récipient bien fermé pour le protéger de la lumière et de l'humidité.
06.5 Nature du conditionnement primaire et contenu de l'emballage
Tube en polypropylène avec bouchon en polyéthylène rempli de gel de silice comme agent desséchant.
Venoruton 500 mg comprimés effervescents: 1 tube de 20 comprimés
Venoruton 1000 mg comprimés effervescents: n. 2 tubes de 15 comprimés chacun.
06.6 Instructions d'utilisation et de manipulation
Les médicaments non utilisés et les déchets dérivés de ce médicament doivent être éliminés conformément aux réglementations locales.
07.0 TITULAIRE DE L'AUTORISATION DE MISE SUR LE MARCHE
Novartis Consumer Health S.p.A., Origgio (Varese).
08.0 NUMÉRO D'AUTORISATION DE MISE SUR LE MARCHÉ
Venoruton 500 mg comprimés effervescents, 20 comprimés : A.I.C. n.m. 017076112
Venoruton 1000 mg comprimés effervescents, 30 comprimés : A.I.C. n.m. 017076124
09.0 DATE DE PREMIÈRE AUTORISATION OU DE RENOUVELLEMENT DE L'AUTORISATION
Date du dernier renouvellement : 1.6.2010
10.0 DATE DE RÉVISION DU TEXTE
Décision AIFA du 16 avril 2013





















-in-alto-abs.jpg)
