Ingrédients actifs : Penciclovir
Crème Vectavir 1%
Pourquoi le Vectavir est-il utilisé ? Pourquoi est-ce?
Vectavir contient le principe actif penciclovir, qui appartient à un groupe de médicaments appelés antiviraux. Le vectavir agit en tuant les virus qui causent l'herpès.
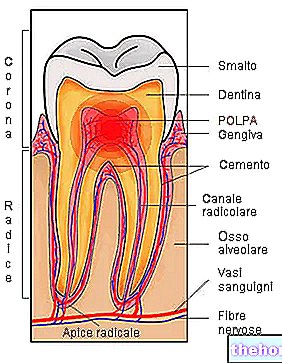
Le Vectavir est indiqué dans le traitement des boutons de fièvre (herpès labial), une maladie infectieuse causée par le virus Herpes Simplex caractérisée par des cloques sur les lèvres remplies d'un liquide clair.
Contre-indications Quand le Vectavir ne doit pas être utilisé
Ne pas utiliser Vectavir
- si vous êtes allergique au penciclovir, au famciclovir ou à l'un des autres composants contenus dans ce médicament (mentionnés dans la rubrique 6)
- si le patient est un enfant de moins de 12 ans.
Précautions d'emploi Quelles sont les informations à connaître avant de prendre Vectavir
Adressez-vous à votre médecin ou pharmacien avant d'utiliser Vectavir.
Consultez votre médecin avant d'utiliser Vectavir :
- si vous êtes enceinte ou si vous allaitez (voir rubrique « Grossesse et allaitement »).
- si votre système immunitaire est très faible, par exemple si vous êtes atteint du SIDA ou avez subi une greffe de moelle osseuse. Votre médecin déterminera si un traitement oral est plus approprié.
Faites particulièrement attention lorsque vous utilisez le Vectavir :
- appliquer la crème uniquement sur les lésions des lèvres et autour de la bouche
- ne pas appliquer la crème sur les muqueuses telles que les yeux, la bouche, le nez ou les organes génitaux
- n'appliquez pas la crème sur ou autour de vos yeux.
Enfants et adolescents
Le vectavir ne doit pas être utilisé chez les enfants de moins de 12 ans
Interactions Quels médicaments ou aliments peuvent modifier l'effet du Vectavir
Informez votre médecin ou pharmacien si vous utilisez, avez récemment utilisé ou pourriez utiliser tout autre médicament, y compris ceux obtenus sans ordonnance.
À ce jour, il n'y a pas d'interactions connues avec d'autres médicaments.
Avertissements Il est important de savoir que :
La grossesse et l'allaitement
Si vous êtes enceinte ou si vous allaitez, si vous pensez être enceinte ou prévoyez une grossesse, demandez conseil à votre médecin ou votre pharmacien avant d'utiliser ce médicament.
Si vous êtes enceinte ou si vous allaitez, n'utilisez Vectavir qu'après avoir consulté votre médecin. Votre médecin déterminera si les bénéfices pour vous l'emportent clairement sur les risques pour le fœtus/l'enfant avant d'utiliser Vectavir.
Conduire et utiliser des machines
À ce jour, il n'y a aucun effet connu sur l'aptitude à conduire des véhicules et à utiliser des machines.
Vectavir contient de l'alcool cétostéarylique : peut provoquer des réactions cutanées locales (par exemple, dermatite de contact)
Vectavir contient du propylène glycol : peut provoquer une irritation cutanée
Dose, mode et heure d'administration Comment utiliser Vectavir : Posologie
Utilisez toujours ce médicament exactement comme décrit dans cette notice ou comme indiqué par votre médecin ou votre pharmacien.
En cas de doute, consultez votre médecin ou votre pharmacien. Utilisation chez les adultes et les enfants de plus de 12 ans
Attention à ne pas dépasser les doses indiquées.
- Appliquer la crème toutes les 2 heures tout au long de la journée (environ 8 fois par jour) pendant 4 jours consécutifs.
- Vous dessinez une petite quantité de crème sur votre doigt et l'appliquez sur la zone infectée.
- Lavez-vous toujours les mains avant d'appliquer la crème.
- Appliquer le Vectavir avec un doigt propre ou un applicateur jetable, s'il est présent dans l'emballage, qui doit être jeté après utilisation.
Utilisez la crème dès que possible, dès les premiers signes d'infection, c'est-à-dire des brûlures et des démangeaisons.
Cependant, même chez les patients qui commencent le traitement plus tard (aux premiers signes de cloques), le Vectavir s'est avéré efficace pour « accélérer la cicatrisation des plaies, réduire la douleur associée et raccourcir le temps de propagation virale ».
N'utiliser que pour de courtes périodes de traitement.
Contactez votre médecin si vous ne vous sentez pas mieux ou si vous vous sentez moins bien après 4 jours, si le trouble survient à plusieurs reprises ou si vous avez remarqué des changements récents dans ses caractéristiques.
Utilisation chez les enfants de moins de 12 ans
Le vectavir ne doit pas être utilisé chez les enfants de moins de 12 ans.
Surdosage Que faire si vous avez pris trop de Vectavir
Si vous avez utilisé plus de Vectavir que vous n'auriez dû
En cas d'ingestion accidentelle ou d'utilisation d'une dose excessive de Vectavir, informez immédiatement votre médecin ou rendez-vous à l'hôpital le plus proche.
Si vous avez appliqué une grande quantité de crème en une seule fois, il est peu probable que vous ressentiez des effets secondaires, mais une "irritation cutanée" peut survenir.
Si vous avez accidentellement ingéré le médicament, il ne devrait pas y avoir d'effets secondaires affectant l'ensemble du corps, une "irritation à l'intérieur de la bouche" pourrait éventuellement se produire.
Si vous oubliez d'utiliser le Vectavir
Ne pas utiliser une double dose pour compenser une dose oubliée.
Si vous oubliez d'utiliser la crème, appliquez-la dès que possible, puis continuez à l'utiliser normalement.
Si vous arrêtez de prendre Vectavir
Si vous avez d'autres questions sur l'utilisation de ce médicament, demandez plus d'informations à votre médecin ou votre pharmacien.
Effets secondaires Quels sont les effets secondaires du Vectavir
Comme tous les médicaments, ce médicament peut provoquer des effets indésirables, bien que tout le monde n'y soit pas sujet.
Effets indésirables fréquents (pouvant affecter jusqu'à 1 personne sur 10)
- réactions dans le domaine d'application, y compris
- sensation de brûlure sur la peau
- douleur cutanée
- diminution de la sensibilité de la peau.
Ces effets sont généralement transitoires.
Effets indésirables de fréquence inconnue (la fréquence ne peut être estimée à partir des données disponibles)
- réactions allergiques, par exemple hypersensibilité et urticaire
- réactions cutanées, par exemple inflammation allergique de la peau (dermatite allergique), y compris des taches rouges sur la peau (éruption cutanée), des démangeaisons, des cloques et un gonflement dû à une accumulation de liquide (œdème).
Le respect des instructions contenues dans la notice réduit le risque d'effets indésirables.
Déclaration des effets secondaires
Si vous ressentez un quelconque effet indésirable, parlez-en à votre médecin ou votre pharmacien, y compris tout effet indésirable éventuel non mentionné dans cette notice. Vous pouvez également signaler les effets secondaires directement via le système national de déclaration à l'adresse https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse
En signalant les effets secondaires, vous pouvez contribuer à fournir plus d'informations sur la sécurité de ce médicament.
Expiration et conservation
Ne pas conserver au dessus de 30°C. Ne pas congeler.
Gardez ce médicament hors de la vue et de la portée des enfants.
N'utilisez pas ce médicament après la date de péremption indiquée sur l'emballage après « EXP ». La date d'expiration fait référence au dernier jour de ce mois.
Ne jetez aucun médicament au tout-à-l'égout ou avec les ordures ménagères.Demandez à votre pharmacien comment jeter les médicaments que vous n'utilisez plus.Cela contribuera à protéger l'environnement.
Il est important de toujours disposer des informations sur le médicament, donc conservez à la fois la boîte et la notice.
ce que contient le Vectavir
- L'ingrédient actif est le penciclovir. Un gramme de crème contient 10 mg de penciclovir.
- Les autres composants sont la paraffine solide, la paraffine liquide, l'alcool cétostéarylique, le propylène glycol, le cétomacrogol 1000 et l'eau purifiée.
A quoi ressemble le Vectavir et contenu de l'emballage extérieur
Le Vectavir se présente sous la forme d'une crème blanche et homogène.
Vectavir est disponible dans les conditionnements suivants :
- Tube de 2 g de crème 1%
- Tube de 2 g en étui rigide avec 20 applicateurs jetables
- flacon avec doseur de 2 g de crème 1%
- Tube de 5 g de crème 1%.
Notice d'emballage source : AIFA (Agence italienne des médicaments). Contenu publié en janvier 2016. Les informations présentes peuvent ne pas être à jour.
Pour avoir accès à la version la plus récente, il est conseillé d'accéder au site Internet de l'AIFA (Agence Italienne du Médicament). Avis de non-responsabilité et informations utiles.
01.0 DÉNOMINATION DU MÉDICAMENT
CRÈME VECTAVIR LABIAL 1%
02.0 COMPOSITION QUALITATIVE ET QUANTITATIVE
Chaque gramme de crème contient 10 mg de penciclovir.
Excipients à effet notoire : 77,2 mg d'alcool cétostéarylique, 416,8 mg de propylène glycol.
Pour la liste complète des excipients, voir rubrique 6.1.
03.0 FORME PHARMACEUTIQUE
Crème.
Crème de couleur beige-marron, d'aspect homogène.
04.0 INFORMATIONS CLINIQUES
04.1 Indications thérapeutiques
VECTAVIR LABIALE est indiqué dans le traitement de l'herpès labial.
04.2 Posologie et mode d'administration
Adultes (y compris les personnes âgées) et les enfants de plus de 12 ans
VECTAVIR LABIAL doit être appliqué à intervalles d'environ 2 heures tout au long de la journée. Le traitement, à poursuivre pendant 4 jours, doit être débuté dès que possible, dès les premiers signes d'infection. Cependant, même chez les patients qui commencent le traitement plus tard. , VECTAVIR LABIAL s'est avéré efficace pour « accélérer la cicatrisation des plaies, réduire la douleur qui y est associée et » raccourcir le temps de propagation virale.
Population pédiatrique
Enfants (moins de 12 ans)
VECTAVIR LABIAL n'est pas recommandé chez les enfants de moins de 12 ans en raison d'un manque de données sur la sécurité et/ou l'efficacité.
04.3 Contre-indications
Hypersensibilité à la substance active, au famciclovir ou à l'un des excipients mentionnés à la rubrique 6.1.
Enfants de moins de 12 ans.
04.4 Mises en garde spéciales et précautions d'emploi appropriées
La crème ne doit être appliquée que sur les lésions des lèvres et autour de la bouche. Il n'est pas recommandé pour une application sur les muqueuses (par exemple dans les yeux, la bouche ou le nez ou sur les organes génitaux). Des précautions particulières doivent être prises pour éviter l'application dans ou près des yeux.
Les patients gravement immunodéprimés (par exemple les patients atteints du SIDA ou ayant subi une greffe de moelle osseuse) doivent être encouragés à consulter un médecin si un traitement oral est indiqué.
VECTAVIR LABIAL contient de l'alcool cétostéarylique : il peut provoquer des réactions cutanées locales (par exemple, dermatite de contact).
VECTAVIR LABIAL contient du propylène glycol : peut provoquer une irritation cutanée.
04.5 Interactions avec d'autres médicaments et autres formes d'interactions
L'expérience des essais cliniques n'a pas identifié d'interactions résultant de l'administration concomitante de médicaments topiques ou systémiques et de la crème VECTAVIR LABIALE.
04.6 Grossesse et allaitement
Grossesse
Lorsque la crème est utilisée chez la femme enceinte, il est peu probable qu'il y ait lieu de s'inquiéter des effets indésirables car l'absorption systémique du penciclovir après application topique de la crème VECTAVIR LABIAL s'est avérée minime (voir rubrique 5.2).
L'innocuité du penciclovir chez la femme enceinte n'ayant pas été établie, VECTAVIR LABIAL crème doit être utilisé sur avis d'un médecin pendant la grossesse ou par les mères qui allaitent, uniquement si les bénéfices potentiels l'emportent sur les risques potentiels associés au traitement.
L'heure du repas
Lorsque la crème est utilisée chez les femmes qui allaitent, il est peu probable qu'il y ait lieu de s'inquiéter des effets indésirables car l'absorption systémique du penciclovir après application topique de la crème VECTAVIR LABIAL s'est avérée minime (voir rubrique 5.2).
Il n'y a aucune information sur l'excrétion du penciclovir dans le lait maternel.
04.7 Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Vectavir Labiale n'affecte pas l'aptitude à conduire des véhicules ou à utiliser des machines.
04.8 Effets indésirables
La crème VECTAVIR LABIAL a été bien tolérée dans les études chez l'homme.L'expérience des essais cliniques a montré qu'il n'y a pas de différence dans la fréquence ou le type d'effets indésirables entre la crème VECTAVIR LABIAL et le placebo.
Les événements les plus courants sont les événements indésirables dans le domaine d'application.
Les effets indésirables sont répertoriés ci-dessous par système organique, classe et fréquence.
Les fréquences sont définies comme suit :
Très commun (>1/10); commun (> 1/100 à peu fréquent (> 1 / 1 000 à rare (> 1 / 10 000 à très rare (inconnu (ne peut être estimé à partir des données disponibles).
Au sein de chaque classe de fréquence, les effets indésirables sont signalés par ordre décroissant de gravité.
La surveillance post-commercialisation a révélé les événements indésirables suivants (toutes les réactions étaient localisées ou générales) : Il est difficile de définir une fréquence pour les événements indésirables post-commercialisation et les événements sont donc répertoriés avec une fréquence inconnue.
Déclaration des effets indésirables suspectés
La déclaration des effets indésirables suspectés survenant après l'autorisation du médicament est importante car elle permet un suivi continu du rapport bénéfice/risque du médicament. Les professionnels de santé sont invités à déclarer tout effet indésirable suspecté via le système national de déclaration. » adresse : www .agenziafarmaco.gov.it/it/responsabili.
04.9 Surdosage
Même après ingestion orale de la totalité du contenu d'une boîte de VECTAVIR LABIAL crème, aucun effet indésirable ne doit survenir ; le penciclovir est mal absorbé après administration orale. Cependant, une irritation de la cavité buccale peut survenir. En cas d'ingestion accidentelle, cela n'est pas nécessaire. pas de traitement particulier.
05.0 PROPRIÉTÉS PHARMACOLOGIQUES
05.1 Propriétés pharmacodynamiques
Groupe pharmacothérapeutique : antibiotiques et agents chimiothérapeutiques à usage dermatologique - antiviraux.
ATC : D06BB06.
Le penciclovir s'est révélé actif in vitro contre les virus de l'herpès simplex (types 1 et 2), la varicelle-zona et Epstein-Barr. Il a également montré une certaine activité in vitro contre le cytomégalovirus. L'activité du penciclovir a également été démontrée dans des modèles animaux contre les infections causées par le virus de l'herpès simplex (type 1 et 2).
Le penciclovir agit sélectivement sur les cellules infectées par le virus, où il est rapidement et efficacement converti en un dérivé triphosphate (conversion médiée par une thymidine kinase codée par le virus). Le dérivé triphosphate reste dans les cellules infectées pendant plus de 12 heures, inhibant la réplication de l'ADN viral. Dans les cellules non infectées traitées au penciclovir, les concentrations de penciclovir triphosphate sont à la limite du seuil de détermination. Par conséquent, il est peu probable que les concentrations thérapeutiques de penciclovir aient un effet sur les cellules non infectées. Avec les analogues nucléosidiques, tels que l'aciclovir, la forme de résistance la plus courante chez les souches d'herpès simplex est un déficit de la production de l'enzyme thymidine kinase (TK).Pour ces souches, une résistance croisée à l'aciclovir et au penciclovir peut être attendue. Cependant, le penciclovir s'est avéré actif contre une souche d'herpès simplex résistante à l'acyclovir récemment isolée, caractérisée par une ADN polymérase altérée.
05.2 "Propriétés pharmacocinétiques
Après application topique de VECTAVIR LABIAL sur peau abrasée et occluse, dans le cadre d'une étude sur volontaires sains, à la dose quotidienne de 180 mg de penciclovir (environ 67 fois la dose thérapeutique habituelle), pendant 4 jours, le penciclovir n'a pas été déterminable dans le plasma ou l'urine.
05.3 Données de sécurité précliniques
Toxicologie générale
L'application topique d'une crème de penciclovir à 5 % sur des rats et des lapins pendant 4 semaines a été bien tolérée.De plus, aucune sensibilisation par contact n'a été observée chez les cobayes.
Les études menées avec le penciclovir administré par voie intraveineuse n'ont révélé aucun problème toxicologique lié à l'application topique du produit.Après administration topique de penciclovir, l'absorption systémique est de toute façon minime.
Génotoxicité et toxicité pour la reproduction
Les résultats des études de mutagénicité, menées à la fois in vitro et in vivo, indiquent que le penciclovir ne présente pas de risque génotoxique pour l'homme.
06.0 INFORMATIONS PHARMACEUTIQUES
06.1 Excipients
Paraffine solide
Paraffine liquide
Alcool cétostéarylique
Propylène glycol
Cétomacrogol 1000
Oxyde de fer rouge (E-172)
Oxyde de fer jaune (E-172)
Eau purifiée
06.2 Incompatibilité
Non pertinent.
06.3 Durée de validité
Tube aluminium de 2 g de crème 3 ans.
Tube aluminium de 5 g de crème 3 ans.
Flacon plastique, avec doseur, de 2 g de crème 18 mois.
06.4 Précautions particulières de conservation
Ne pas congeler.
Ne pas conserver au dessus de 30°C.
06.5 Nature du conditionnement primaire et contenu de l'emballage
1 tube aluminium de 2 g de crème 1%
1 flacon plastique, avec doseur, de 2 g de crème 1%
1 tube aluminium de 5 g de crème 1%
Toutes les présentations peuvent ne pas être commercialisées.
06.6 Instructions d'utilisation et de manipulation
Les médicaments non utilisés et les déchets dérivés de ce médicament doivent être éliminés conformément aux réglementations locales.
07.0 TITULAIRE DE L'AUTORISATION DE MISE SUR LE MARCHE
Novartis Consumer Health S.p.A. - Largo Umberto Boccioni, 1 - 21040 Origgio (VA)
08.0 NUMÉRO D'AUTORISATION DE MISE SUR LE MARCHÉ
1 tube de 2 g de crème 1% A.I.C. n.m. 032154015
1 flacon, avec doseur, de 2 g de crème 1% A.I.C. n.m. 032154027
1 tube de 5 g de crème 1% A.I.C. n.m. 032154039
09.0 DATE DE PREMIÈRE AUTORISATION OU DE RENOUVELLEMENT DE L'AUTORISATION
17.02.1998 / 27.02.2008
10.0 DATE DE RÉVISION DU TEXTE
juin 2015