Ingrédients actifs : Thiétylpérazine (maléate de thiétylpérazine)
Torecan 6,5 mg comprimés enrobés
Suppositoires Torecan 6,5 mg
Indications Pourquoi utiliser Torecan ? Pourquoi est-ce?
CATÉGORIE PHARMACOTHERAPEUTIQUE
Dérivé de phénothiazine, à activité antiémétique.
INDICATIONS THÉRAPEUTIQUES
Nausées et vomissements induits par les agents chimiothérapeutiques antiblastiques, la radiothérapie, les agents toxiques et la chirurgie.
Contre-indications Quand Torecan ne doit pas être utilisé
Hypersensibilité à la substance active, à l'un des excipients ou aux phénothiazines.
États comateux et états dépressifs sévères.
Troubles de l'hématopoïèse.
Maladies du foie.
Enfants de moins de 15 ans (voir "Précautions d'emploi" et "Effets indésirables").
Grossesse (voir "Mises en garde spéciales - Grossesse")
Allaitement (voir « Mises en garde spéciales – Allaitement ».)
Précautions d'emploi Quelles sont les informations à connaître avant de prendre Torecan
Comme avec tous les neuroleptiques, les patients traités doivent être maintenus sous surveillance médicale directe, en particulier ceux qui ont précédemment présenté une sensibilité anormale aux phénothiazines. (voir « Effets indésirables »).
Torecan est contre-indiqué chez les enfants de moins de 15 ans car cette tranche d'âge est particulièrement sujette aux effets secondaires extrapyramidaux et aux convulsions (voir « Contre-indications » et « Effets indésirables »).
Une attention particulière doit être portée aux patients présentant un phéochromocytome ou une insuffisance mitrale pour tout effet hypotenseur pouvant survenir. Les épisodes d'hypotension avec Torecan sont rares (voir « Effets indésirables »); cependant, s'ils surviennent, ils peuvent être contrôlés par la noradrénaline, l'angiotensine ou la phényléphrine (pas l'adrénaline, dont l'action peut être contrariée par les phénothiazines).
Une élévation notable de la température corporelle peut être l'expression d'une réaction idiosyncratique et le traitement doit donc être arrêté.
Les phénothiazines peuvent augmenter l'état de raideur musculaire chez les individus prédisposés ou déjà atteints de la maladie de Parkinson ou de formes de type Parkinson, ou d'autres troubles moteurs.
Chez les patients insuffisants rénaux et chez les patients présentant des troubles du système nerveux central, le produit peut induire des manifestations neurotoxiques, à des doses supérieures à celles recommandées.
Traitement à long terme
Dans de rares cas, des dyskinésies tardives ont été observées après un traitement à long terme par Torecan chez des patients âgés (voir « Effets indésirables »). Les patients de ce groupe d'âge doivent donc être traités pendant la période la plus courte possible et doivent être étroitement surveillés afin de détecter la survenue d'événements neurologiques nocifs.
Phase post-opératoire
En cas d'administration de Torecan pendant la phase post-opératoire de l'anesthésie, la survenue éventuelle d'une dépression et/ou d'une agitation du système nerveux central doit être prise en compte.
Insuffisance hépatique
La prudence est recommandée chez les patients présentant une insuffisance hépatique légère, modérée et sévère ou une cirrhose (voir « Dose, mode et moment d'administration » et « Effets indésirables »).
Chez les patients qui fument 20 cigarettes ou plus par jour, l'utilisation prolongée de Torecan 6,5 mg comprimés enrobés peut augmenter le risque de développer un cancer du poumon.
Interactions Quels médicaments ou aliments peuvent modifier l'effet de Torecan
Médicaments qui agissent sur le S.N.C.
Informez votre médecin ou votre pharmacien si vous avez récemment pris tout autre médicament, même sans ordonnance.
L'association avec d'autres psychotropes requiert une prudence particulière.
Les phénothiazines pouvant accentuer l'action dépressive sur le système nerveux central des opiacés, des antihistaminiques, des analgésiques, des benzodiazépines et des barbituriques, la posologie de ces médicaments, s'ils sont utilisés en même temps, doit être convenablement adaptée.
La sensibilité à l'alcool, à l'atropine et aux insecticides phosphoriques est accentuée lors du traitement par les phénothiazines.
Torecan ne doit être utilisé qu'en cas de stricte nécessité et avec une extrême prudence en association avec des médicaments dépresseurs, le S.N.C. indiqué ci-dessus.
Inhibiteurs du CYP2D6
L'administration concomitante d'inhibiteurs du CYP2D6 (tels que la fluoxétine et la quinidine) peut augmenter les concentrations plasmatiques de la thylpérazine.
Anticonvulsivants
Torecan peut diminuer l'efficacité des anticonvulsivants en raison des propriétés épileptogènes des phénothiazines.
En conjonction avec l'administration de Torecan, si le patient est traité avec un anticonvulsivant, une dose plus élevée de ce médicament peut être nécessaire.
Avertissements Il est important de savoir que :
Grossesse
Demandez conseil à votre médecin ou votre pharmacien avant de prendre tout médicament
Il n'existe que des données limitées sur l'utilisation de la thiétylpérazine chez les patientes enceintes. Par mesure de précaution, Torecan ne doit pas être utilisé pendant la grossesse.
L'heure du repas
Les phénothiazines étant excrétées dans le lait maternel, Torecan ne doit pas être utilisé pendant l'allaitement (voir « Contre-indications »).
La fertilité
La fertilité des rats mâles n'a pas été affectée par le traitement à la thiétylpérazine. Des effets sur le taux de gestation des rates n'ont été observés qu'à des expositions considérées comme suffisamment supérieures à l'exposition humaine maximale, ce qui indique peu de pertinence pour l'utilisation clinique.
Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Les patients doivent être informés que des réactions indésirables du système nerveux central telles qu'une somnolence et des symptômes extrapyramidaux (dystonie, crise oculogyre, spasmes musculaires) peuvent survenir pendant le traitement par la thiétylpérazine. Par conséquent, il convient de faire preuve de prudence lors de la conduite d'un véhicule ou de l'utilisation de machines.
Informations importantes sur certains ingrédients
Les comprimés enrobés de Torecan contiennent du lactose et du saccharose. Par conséquent, si votre médecin vous a diagnostiqué une intolérance à certains sucres, contactez votre médecin avant de prendre ce médicament.
En raison de la présence de bêta-carotène dans la composition, l'utilisation prolongée de comprimés enrobés de Torecan peut augmenter le risque de développer un cancer du poumon chez les gros fumeurs (20 cigarettes ou plus par jour).
Posologie et mode d'utilisation Comment utiliser Torecan : Posologie
DOSAGE
Population générale
1 comprimé enrobé ou 1 suppositoire 1 à 3 fois par jour.
Populations particulières
Insuffisance rénale Aucune étude n'a été réalisée chez des patients atteints d'insuffisance rénale.
Insuffisance hépatique
Chez les patients insuffisants hépatiques prenant Torecan à fortes doses ou pendant une période prolongée, la fonction hépatique doit être surveillée (voir "Précautions d'emploi" et "Effets indésirables").
Population pédiatrique
Torecan est contre-indiqué chez l'enfant de moins de 15 ans (voir « Contre-indications », « Précautions d'emploi » et « Effets indésirables »).
personnes agées
Torecan doit être utilisé avec prudence chez les patients âgés (65 ans et plus) (voir « Précautions d'emploi »).
MODE D'ADMINISTRATION
Les comprimés enrobés doivent être pris à jeun, uniquement pendant de courtes périodes et avec de grands intervalles entre ces périodes.
En raison de son goût désagréable, les comprimés enrobés de Torecan ne doivent pas être mâchés. Les suppositoires Torecan doivent être bien insérés dans le rectum. Il est recommandé de prendre le suppositoire après avoir passé les selles. Les suppositoires ne doivent pas être pris par voie orale. Pour usage rectal uniquement.
Surdosage Que faire si vous avez pris trop de Torecan
Symptômes:
Engourdissements, états confusionnels suivis dans les cas les plus sévères de coma et perte de réflexes ; tachycardie, hypotension orthostatique, collapsus ; dépression respiratoire; agitation, réactions dystoniques aiguës, convulsions.
Traitement:
Il n'y a pas d'antidote. Les mesures générales de soutien comprennent un lavage gastrique suivi de l'administration de charbon activé et, si nécessaire, une surveillance des fonctions cardiovasculaire et respiratoire.
Traitements symptomatiques :
Hypotension aiguë : administrer des extenseurs plasmatiques ; s'il est nécessaire d'administrer un médicament vasopresseur (pas d'adrénaline), la fonction cardiovasculaire doit être étroitement surveillée.
Réactions dystoniques aiguës : administrer des médicaments anti-parkinsoniens.
Convulsions : administrer des benzodiazépines.
En cas d'ingestion accidentelle / prise d'une dose excessive de Torecan, prévenez immédiatement votre médecin ou rendez-vous à l'hôpital le plus proche
Effets secondaires Quels sont les effets secondaires de Torecan
Effets indésirables observés après administration de phénothiazines
Comme tous les médicaments, Torecan est susceptible d'avoir des effets indésirables, bien que tout le monde n'y soit pas sujet.
La thiétylpérazine (Torecan) est un dérivé de la phénothiazine. Le médecin doit savoir que les effets secondaires suivants sont survenus avec une ou plusieurs phénothiazines :
- Réactions allergiques : avec les phénothiazines elles ont été exceptionnellement rapportées et caractérisées par des phénomènes cutanés localisés ou étendus (plus rarement il s'agit d'idiosyncrasies avec fièvre) tels qu'érythème, prurit, urticaire, eczéma, œdème localisé, phénomènes de photosensibilisation, dermatite exfoliative. Les crises d'asthme sont rares.
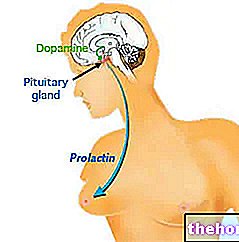
- Autres manifestations : insomnie légère, états d'excitation paradoxale, hypotension artérielle, tachycardie, bouche sèche, congestion nasale, constipation, vision trouble, augmentation des taux de prolactine.
- Bien qu'à une fréquence extrêmement faible, des lésions du foie avec ictère et de la vascularisation sanguine avec agranulocytose et thrombocytopénie ont été décrites suite à un traitement par phénothiazines.
Effets indésirables observés après administration de Torecan
Les effets indésirables suivants sont répertoriés par système/organe de classe MedDRA.
Au sein de chaque organe/classe, les effets indésirables sont classés par fréquence, en commençant par les effets les plus fréquents.Au sein de chaque classe de fréquence, les effets indésirables sont classés par ordre décroissant de gravité. De plus, la catégorie de fréquence de chaque effet indésirable est basée sur la convention suivante (CIOMS III) : très fréquent (≥1/10) ; commun (≥1 / 100,
Effets indésirables
Troubles du système immunitaire
Très rare : Réactions anaphylactiques ou anaphylactoïdes (voir "Précautions d'emploi")
Troubles du système nerveux
Rare : Symptômes extrapyramidaux*, crise oculogyre, dyskinésie tardive (voir "Précautions d'emploi"), somnolence, dysarthrie
Très rare : syndrome malin des neuroleptiques
Pathologies cardiaques
Rare : Hypotension (voir "Précautions d'emploi")
Très rare : Tachycardie
Problèmes gastro-intestinaux
Rare : Dysphagie
Très rare : Bouche sèche
Troubles hépatobiliaires
Très rare : Anomalies de la fonction hépatique
Affections de la peau et du tissu sous-cutané
Très rare : Dermatite (voir "Précautions d'emploi")
Troubles musculo-squelettiques et du tissu conjonctif
Très rare : Spasmes musculaires
* Comme les autres dérivés de la phénothiazine, Torecan peut - bien que rarement et généralement chez les patients plus jeunes - provoquer des symptômes extrapyramidaux (crise oculogyre, difficultés à avaler et à parler, spasmes musculaires, trismus). Ces symptômes répondent généralement rapidement à un traitement parentéral avec un agent antiparkinsonien. Dans de nombreux cas, les symptômes extrapyramidaux disparaissent rapidement avec l'arrêt du médicament.
Effets indésirables de l'expérience post-commercialisation
L'effet indésirable suivant a été déduit de l'expérience post-commercialisation avec Torecan. Comme cet effet est signalé volontairement à partir d'une population de taille indéfinie, il n'est pas possible d'estimer de manière fiable sa fréquence qui est donc classée comme inconnue.
Fréquence inconnue
Affections de la peau et du tissu sous-cutané
Oedème de Quincke
Le respect des instructions contenues dans la notice réduit le risque d'effets indésirables.
Déclaration des effets secondaires
Si vous ressentez un quelconque effet indésirable, parlez-en à votre médecin ou votre pharmacien, y compris tout effet indésirable éventuel non mentionné dans cette notice. Les effets indésirables peuvent également être signalés directement via le système national de notification à l'adresse "https://www.aifa.gov.it/content/segnalazioni-reazioni-avverse". En signalant les effets secondaires, vous pouvez contribuer à fournir plus d'informations sur la sécurité de ce médicament.
Expiration et conservation
Expiration : voir la date d'expiration imprimée sur l'emballage.
La date de péremption indiquée fait référence au produit dans un emballage intact, correctement stocké.
La date de péremption fait référence au dernier jour du mois.
Attention : ne pas utiliser le médicament après la date de péremption indiquée sur l'emballage.
Les médicaments ne doivent pas être jetés au tout-à-l'égout ou avec les ordures ménagères.
Demandez à votre pharmacien comment jeter les médicaments que vous n'utilisez plus. Cela contribuera à protéger l'environnement.
Gardez ce médicament hors de la vue et de la portée des enfants.
Composition et forme pharmaceutique
Des comprimés enrobés
1 comprimé enrobé contient :
Principe actif :
maléate de thiétylpérazine 10,276 mg (équivalent à 6,5 mg de base)
Excipients : gomme arabique, gélatine, talc, acide stéarique, amidon de maïs, lactose, saccharose, -carotène, huile d'arachide.
Suppositoires
1 suppositoire contient :
Principe actif :
maléate de thiétylpérazine 10,276 mg (équivalent à 6,5 mg de base)
Excipients : Lactose monohydraté, glycérides semi-synthétiques solides.
FORME PHARMACEUTIQUE et CONTENU
Torecan 6,5 mg comprimés enrobés : 15 comprimés enrobés
Suppositoires Torecan 6,5 mg : 6 suppositoires
Notice d'emballage source : AIFA (Agence italienne des médicaments). Contenu publié en janvier 2016. Les informations présentes peuvent ne pas être à jour.
Pour avoir accès à la version la plus récente, il est conseillé d'accéder au site Internet de l'AIFA (Agence Italienne du Médicament). Avis de non-responsabilité et informations utiles.
01.0 DÉNOMINATION DU MÉDICAMENT
TORÉCAN
02.0 COMPOSITION QUALITATIVE ET QUANTITATIVE
Des comprimés enrobés
1 comprimé enrobé contient :
Principe actif:
maléate de thiéthylpérazine ....................................................... ................................... 10,276 mg
(égal à 6,5 mg de thiétylpérazine base).
Excipients : lactose, saccharose
Suppositoires
1 suppositoire contient :
Principe actif:
maléate de thiéthylpérazine ....................................................... ................................... 10,276 mg
(égal à 6,5 mg de thiétylpérazine base).
Pour la liste complète des excipients, voir rubrique 6.1.
03.0 FORME PHARMACEUTIQUE
Des comprimés enrobés.
Suppositoires.
04.0 INFORMATIONS CLINIQUES
04.1 Indications thérapeutiques
Nausées et vomissements induits par les agents chimiothérapeutiques antiblastiques, la radiothérapie, les agents toxiques et la chirurgie.
04.2 Posologie et mode d'administration
DOSAGE
Population générale
1 comprimé enrobé ou 1 suppositoire 1 à 3 fois par jour.
Populations particulières
Insuffisance rénale
Aucune étude n'a été réalisée chez des patients atteints d'insuffisance rénale.
Insuffisance hépatique
Chez les patients insuffisants hépatiques prenant Torecan à fortes doses ou pendant une période prolongée, la fonction hépatique doit être surveillée (voir rubriques 4.4 et 4.8).
Population pédiatrique
Torecan est contre-indiqué chez les enfants de moins de 15 ans (voir rubriques 4.3, 4.4 et 4.8).
personnes agées
Torecan doit être utilisé avec prudence chez les patients âgés (65 ans et plus) (voir rubrique 4.4).
MODE D'ADMINISTRATION
En raison de son goût désagréable, les comprimés enrobés de Torecan ne doivent pas être mâchés.
Les comprimés enrobés doivent être pris à jeun, uniquement pendant de courtes périodes de traitement et avec de grands intervalles entre ces périodes.
Les suppositoires Torecan doivent être bien insérés dans le rectum. Il est recommandé de prendre le suppositoire après avoir passé les selles. Les suppositoires ne doivent pas être pris par voie orale. Pour usage rectal uniquement.
04.3 Contre-indications
Hypersensibilité à la substance active, à l'un des excipients ou aux phénothiazines.
États comateux et états dépressifs sévères.
Troubles de l'hématopoïèse.
Maladies du foie.
Enfants de moins de 15 ans (voir rubriques 4.4 et 4.8).
Grossesse (voir rubrique 4.6)
Allaitement (voir rubrique 4.6)
04.4 Mises en garde spéciales et précautions d'emploi appropriées
Comme pour tous les neuroleptiques, les patients traités doivent être placés sous surveillance médicale directe, en particulier ceux qui ont précédemment présenté une sensibilité anormale aux phénothiazines (voir 4.8).
Torecan est contre-indiqué chez les enfants de moins de 15 ans car cette tranche d'âge est particulièrement sujette aux effets indésirables extrapyramidaux et aux convulsions (voir rubriques 4.3 et 4.8).
Une attention particulière doit être portée aux patients présentant un phéochromocytome ou une insuffisance mitrale pour tout effet hypotenseur pouvant survenir. Les épisodes d'hypotension avec Torecan sont rares (voir rubrique 4.8) ; cependant, s'ils surviennent, ils peuvent être contrôlés par la noradrénaline, l'angiotensine ou la phényléphrine (pas l'adrénaline, dont l'action peut être contrariée par les phénothiazines).
Une élévation notable de la température corporelle peut être l'expression d'une réaction idiosyncratique et le traitement doit donc être arrêté.
Les phénothiazines peuvent augmenter l'état de raideur musculaire chez les individus prédisposés ou déjà atteints de la maladie de Parkinson ou de formes de type Parkinson ou d'autres troubles moteurs.
Chez les patients insuffisants rénaux et chez les patients présentant des troubles du système nerveux central, le produit peut induire des manifestations neurotoxiques, à des doses supérieures à celles recommandées.
Traitement à long terme
Dans de rares cas, des dyskinésies tardives ont été observées après un traitement à long terme par Torecan chez des patients âgés (voir 4.8). Les patients de ce groupe d'âge doivent donc être traités pendant la période la plus courte possible et doivent être étroitement surveillés afin de détecter la survenue d'événements neurologiques nocifs.
Phase post-opératoire
En cas d'administration de Torecan pendant la phase post-opératoire de l'anesthésie, la survenue éventuelle d'une dépression et/ou d'une agitation du système nerveux central doit être prise en compte.
Insuffisance hépatique
La prudence est recommandée chez les patients présentant une insuffisance hépatique légère, modérée et sévère ou une cirrhose (voir rubriques 4.2, 4.8 et 5.1 - « Populations particulières »).
Informations importantes sur certains ingrédients
Les comprimés enrobés de Torecan contiennent lactose, par conséquent, les patients présentant des problèmes héréditaires rares d'intolérance au galactose, de déficit en lactase de Lapp ou de malabsorption du glucose/galactose ne doivent pas prendre ce médicament.
Les comprimés enrobés de Torecan contiennent saccharose, par conséquent, les patients présentant des problèmes héréditaires rares d'intolérance au fructose, de malabsorption du glucose/galactose ou d'insuffisance en sucrase isomaltase ne doivent pas prendre ce médicament.
En raison de la présence de bêta-carotène dans la composition, l'utilisation prolongée de comprimés enrobés de Torecan peut augmenter le risque de développer un cancer du poumon chez les gros fumeurs (20 cigarettes ou plus par jour).
04.5 Interactions avec d'autres médicaments et autres formes d'interactions
Interactions à considérer
Médicaments qui agissent sur le SNC
L'association avec d'autres psychotropes requiert une prudence particulière.
Les phénothiazines pouvant accentuer l'action dépressive sur le système nerveux central des opiacés, des antihistaminiques, des analgésiques, des benzodiazépines et des barbituriques, la posologie de ces médicaments, en cas d'utilisation concomitante, devra être adaptée en conséquence.
La sensibilité à l'alcool, à l'atropine et aux insecticides phosphoriques est accentuée lors du traitement par les phénothiazines.
Torecan ne doit être utilisé qu'en cas de stricte nécessité et avec une extrême prudence en association avec des médicaments dépresseurs, le S.N.C. indiqué ci-dessus.
Inhibiteurs du CYP2D6
L'administration concomitante d'inhibiteurs du CYP2D6 (tels que la fluoxétine et la quinidine) peut augmenter les concentrations plasmatiques de la thylpérazine.
Anticonvulsivants
Torecan peut diminuer l'efficacité des anticonvulsivants en raison des propriétés épileptogènes des phénothiazines.
En conjonction avec l'administration de Torecan, si le patient est traité avec un anticonvulsivant, une dose plus élevée de ce médicament peut être nécessaire.
04.6 Grossesse et allaitement
Grossesse
Seules des données limitées sont disponibles sur l'utilisation de la thiétylpérazine chez les patientes enceintes. Les études chez l'animal sont insuffisantes pour exclure un effet tératogène de Torecan (voir 5.3), et une association possible a été trouvée dans deux études observationnelles. Par mesure de précaution, Torecan doit ne pas être utilisé pendant la grossesse.
L'heure du repas
Les phénothiazines étant excrétées dans le lait maternel, Torecan ne doit pas être utilisé pendant l'allaitement (voir rubrique 4.3).
La fertilité
La fertilité des rats mâles n'a pas été affectée par le traitement à la thiétylpérazine. Des effets sur le taux de gestation des rates n'ont été observés qu'à des expositions considérées comme suffisamment supérieures à l'exposition humaine maximale, ce qui indique peu de pertinence pour l'utilisation clinique.
04.7 Effets sur l'aptitude à conduire des véhicules et à utiliser des machines
Les patients doivent être informés que des réactions indésirables du système nerveux central telles qu'une somnolence et des symptômes extrapyramidaux (dystonie, crise oculogyre, spasmes musculaires) peuvent survenir pendant le traitement par la thiétylpérazine. Par conséquent, il convient de faire preuve de prudence lors de la conduite d'un véhicule ou de l'utilisation de machines.
04.8 Effets indésirables
Effets indésirables observés après administration de phénothiazines
La thiétylpérazine (Torecan) est un dérivé de la phénothiazine. Le médecin doit savoir que les effets secondaires suivants sont survenus avec une ou plusieurs phénothiazines :
• Réactions allergiques : avec les phénothiazines elles ont été exceptionnellement rapportées et caractérisées par des phénomènes cutanés localisés ou diffus (plus rarement il s'agit d'idiosyncrasies avec fièvre) tels qu'érythème, prurit, urticaire, eczéma, œdème localisé, phénomènes de photosensibilisation, dermatite exfoliative. Les crises d'asthme sont rares.
• Autres manifestations : insomnie légère, états d'excitation paradoxale, hypotension artérielle, tachycardie, bouche sèche, congestion nasale, constipation, vision trouble, augmentation des taux de prolactine.
• Bien qu'à une fréquence extrêmement faible, des lésions du foie avec ictère et de la vascularisation sanguine avec agranulocytose et thrombocytopénie ont été décrites suite à un traitement par phénothiazines.
Effets indésirables observés après administration de Torecan
Les effets indésirables suivants (tableau 1.1) sont répertoriés par système/organe de classe MedDRA. Au sein de chaque organe/classe, les effets indésirables sont classés par fréquence, en commençant par les effets les plus fréquents.Au sein de chaque classe de fréquence, les effets indésirables sont classés par ordre décroissant de gravité. De plus, la catégorie de fréquence de chaque effet indésirable est basée sur la convention suivante (CIOMS III) : très fréquent (≥1/10) ; commun (≥1 / 100,
Tableau 1.1 Effets indésirables
* .... Comme les autres dérivés des phénothiazines, Torecan peut - bien que rarement et chez des patients plus jeunes - provoquer des symptômes extrapyramidaux (crise oculogyre, difficultés à avaler et à parler, spasmes musculaires, trismus). Ces symptômes répondent généralement rapidement à un traitement parentéral avec un agent antiparkinsonien. Dans de nombreux cas, les symptômes extrapyramidaux disparaissent rapidement avec l'arrêt du médicament.
Effets indésirables de l'expérience post-commercialisation
L'effet indésirable suivant (tableau 1.2) a été dérivé de l'expérience post-commercialisation avec Torecan. Étant donné que cet effet est signalé volontairement à partir d'une population de taille indéfinie, il n'est pas possible d'estimer de manière fiable sa fréquence qui est donc classée comme non notable. sont répertoriés par classe de système d'organe dans MedDRA. Au sein de chaque classe de système d'organe, les effets indésirables sont répertoriés par ordre décroissant de gravité.
Tableau 1.2 Effets indésirables (fréquence indéterminée)
Déclaration des effets indésirables suspectés
La déclaration des effets indésirables suspectés survenant après autorisation du médicament est importante car elle permet un suivi continu du rapport bénéfice/risque du médicament. Les professionnels de santé sont invités à déclarer tout effet indésirable suspecté via le système national de déclaration. agenziafarmaco.gov.it/it/responsabili.
04.9 Surdosage
Symptômes:
Engourdissements, états confusionnels suivis dans les cas les plus sévères de coma et perte de réflexes ; tachycardie, hypotension orthostatique, collapsus ; dépression respiratoire; agitation, réactions dystoniques aiguës, convulsions.
Traitement:
Il n'y a pas d'antidote. Les mesures générales de soutien comprennent un lavage gastrique suivi de l'administration de charbon activé et, si nécessaire, une surveillance des fonctions cardiovasculaire et respiratoire.
Traitements symptomatiques:
Hypotension aiguë : administrer des extenseurs plasmatiques ; s'il est nécessaire d'administrer un médicament vasopresseur (pas d'adrénaline), la fonction cardiovasculaire doit être étroitement surveillée.
Réactions dystoniques aiguës : administrer des médicaments anti-parkinsoniens.
Convulsions : administrer des benzodiazépines.
05.0 PROPRIÉTÉS PHARMACOLOGIQUES
05.1 Propriétés pharmacodynamiques
Classe pharmacothérapeutique : antiémétiques et antinauséeux.
Code ATC : A04AD49.
Mécanisme d'action
La thiétylpérazine (Torecan) est un dérivé de la phénothiazine qui présente les mêmes caractéristiques pharmacologiques de cette classe chimique de médicaments, dans une mesure plus atténuée, mais a, vis-à-vis de celles-ci, une efficacité spécifique.
Torecan est efficace dans le traitement des nausées et vomissements d'origines diverses.
L'effet antiémétique est dû à sa double action sur la zone bulbaire chimiosensible et sur le centre émétique proprement dit.
Aux doses thérapeutiques, Torecan n'a pas d'effet sédatif-hypnogène.
05.2 Propriétés pharmacocinétiques
Absorption
La thiétylpérazine est bien absorbée par le tractus gastro-intestinal. Après administration orale, la concentration plasmatique maximale de thiétylpérazine est atteinte en 2 à 4 heures.
Distribution
Le volume de distribution a été calculé comme étant de 2,7 L/kg de poids corporel. La demi-vie d'élimination est d'environ 12 heures. La thiétylpérazine peut traverser la barrière placentaire.
Biotransformation / Métabolisme
La thiétylpérazine subit un métabolisme hépatique important.
Élimination
Environ 3 % de la dose administrée sont excrétés sous forme inchangée dans les urines.
Populations particulières
Insuffisance hépatique
La prudence est recommandée chez les patients présentant une insuffisance hépatique légère, modérée et sévère ou une cirrhose en raison de l'augmentation possible de l'exposition à la thiétylpérazine.
Insuffisance rénale
Aucune information n'est disponible sur l'effet de l'insuffisance rénale sur la thiétylpérazine.
05.3 Données de sécurité précliniques
Dans les études non cliniques, des effets n'ont été observés qu'à des expositions considérées comme significativement supérieures à l'exposition humaine maximale, ce qui suggère peu de pertinence clinique.
Études de toxicité
Mutagénicité
La thiétylpérazine n'a montré aucun potentiel mutagène dans un test de mutagénicité bactérienne utilisant Salmonelle typhimurium Et Escherichia coli.
Carcinogenèse
Le potentiel cancérigène de la thiétylpérazine n'a pas été évalué.
Toxicité pour la reproduction
Aucun signe de tératogénicité ou de toxicité pour le développement sur la reproduction n'a été observé chez le rat et le lapin, mais les études présentaient des limites. A doses élevées, toxiques pour la mère et largement supérieures à la dose clinique, une incidence plus élevée de fente palatine a été rapportée chez la souris (50 mg/kg/jour) et le rat (200 mg/kg/jour). La pertinence de cette observation pour l'utilisation clinique de la thiétylpérazine pendant la grossesse humaine est inconnue.
06.0 INFORMATIONS PHARMACEUTIQUES
06.1 Excipients
Comprimés enrobés : gomme arabique, gélatine, talc, acide stéarique, amidon de maïs, lactose, saccharose, -carotène, huile d'arachide.
Suppositoires : lactose monohydraté, glycérides semi-synthétiques solides.
06.2 Incompatibilité
Rien.
06.3 Durée de validité
Comprimés enrobés : 5 ans.
Suppositoires : 5 ans.
06.4 Précautions particulières de conservation
Ce médicament ne nécessite pas de conditions particulières de conservation.
06.5 Nature du conditionnement primaire et contenu de l'emballage
Blister PVC opaque contenant 15 comprimés enrobés.
Bande PVC/PE contenant 6 suppositoires.
06.6 Instructions d'utilisation et de manipulation
Pas d'instructions particulières.
07.0 TITULAIRE DE L'AUTORISATION DE MISE SUR LE MARCHE
Sandoz S.p.A.
Largo Umberto Boccioni, 1 - 21040 Origgio (VA)
08.0 NUMÉRO D'AUTORISATION DE MISE SUR LE MARCHÉ
Torecan 6,5 mg comprimés enrobés .................................. A.I.C. n.m. 019889043
Torecan 6,5 mg suppositoires A.I.C. n.m. 019889031
09.0 DATE DE PREMIÈRE AUTORISATION OU DE RENOUVELLEMENT DE L'AUTORISATION
Première autorisation: 15.05.1962
Renouvellement: 01.06.2010
10.0 DATE DE RÉVISION DU TEXTE
12/07/2015









.jpg)